|
|
 |
 |
 |
 |
|
|
Treatment of Acquired Pendular Nystagmus from Multiple Sclerosis with Eye Muscle Surgery Followed by Oral Memantine
Digital Journal of Ophthalmology 2005
Volume 11, Number 4
August 3, 2005
|
Printer Friendly
|


 Robert L. Tomsak, MD, PhD
Robert L. Tomsak, MD, PhD | Daroff-Dell'Osso Ocular Motility Laboratory, Department of Neurology and Ophthalmology, Louis Stokes Cleveland Department of VA Medical Center, Case Western Reserve University Louis F. Dell'Osso, PhD | Daroff-Dell\'Osso Ocular Motility Laboratory, Department of Neurology and Biomedical Engineering, Louis Stokes Cleveland Department of VA Medical Center, Case Western Reserve University Janet C. Rucker, MD | Daroff-Dell'Osso Ocular Motility Laboratory, Department of Neurology and Ophthalmology, Louis Stokes Cleveland Department of VA Medical Center, Case Western Reserve University R. John Leigh, MD | Daroff-Dell'Osso Ocular Motility Laboratory, Department of Neurology, Biomedical Engineering, and Neurosciences, Louis Stokes Cleveland Department of VA Medical Center, Case Western Reserve University Don C. Bienfang, MD | Brigham and Women’s Hospital Jonathan B. Jacobs, PhD | Daroff-Dell'Osso Ocular Motility Laboratory, Department of Neurology, Louis Stokes Cleveland Department of VA Medical Center, Case Western Reserve University
|
|
|
| Abstract | Purpose. We report the ocular motor and visual acuity effects of the first use of the tenotomy procedure on different types of acquired pendular nystagmus (APN) in two patients with multiple sclerosis (MS) and intractable oscillopsia and of tenotomy plus memantine in one patient.
Methods. (Case 1) Medial rectus muscles of both eyes were tenotomized and reattached and lateral rectus muscles recessed to correct exotropia; memantine was given following surgery. Search-coils were used to study fixation, saccades, pursuit and the OKR and VOR before and after surgery and after memantine. Nystagmus velocities, amplitudes, frequencies, and biplanar eXpanded Nystagmus Acuity Function (NAFX) values were determined. (Case 2) Pre- and post-tenotomy eye movements were studied by digitized video recordings. Horizontal rectus muscles of only the eye with APN were tenotomized and reattached.
Results. (Case 1) Following surgery, APN decreased by ~50% and NAFX values increased by 34%. Measured Snellen visual acuity increased 100% from 0.125 OD and OS to 0.25. Saccades were unaffected. Memantine further damped the APN 69% and increased the NAFX by 9%. Visual acuity increased 60% to 0.4. The cumulative effects were: APN reduced by 82%; NAFX increased by 46%; acuity increased by 220%; and oscillopsia reduced by 75%. (Case 2) Two-muscle tenotomy OD reduced uniocular nystagmus by 66%. Visual acuity increased 100% from 0.2 to 0.4.
Conclusions. Four-muscle surgery (including tenotomy and reattachment) reduced APN and oscillopsia and improved visual acuity; memantine provided additional improvement. Their synergistic effect suggests a dual-mode (surgery + drug) therapy for maximal effectiveness in APN. Two-muscle tenotomy and reattachment was sufficient to damp uniocular APN. | | | Introduction | Acquired pendular nystagmus (APN), encountered in disorders of central myelin, including multiple sclerosis (MS),(1) is often resistant to treatment and causes excessive retinal image motion, reduced visual acuity, and oscillopsia (illusory motion). Some patients respond to gabapentin but others do not or cannot tolerate side effects.(2, 3) In the few patients exhibiting a “null region,” extraocular muscle surgery (Anderson-Kestenbaum) similar to that used in infantile nystagmus syndrome (INS) (4) has been tried. It has been used alone (5) or in combination with gabapentin. (6) More recently, aggressive bilateral eight-muscle surgery (recessions, myectomies, and tenectomies) was applied to acquired nystagmus and strabismus, (7) mimicking the common use of both nystagmus and strabismus surgery in INS. Tenotomy and reattachment was first shown to suppress infantile nystagmus (IN) in a canine model, (8) and later in a formal, masked-data, human clinical trial on INS patients.(9, 10) Its effects were immediate and long lasting. Because the surgery acts independently of the source of the nystagmus, it was hypothesized to be effective in acquired nystagmus.(8, 11) Memantine was reported to be effective in treating APN secondary to MS. (12)
We describe the first applications of the tenotomy procedure to alleviate APN and its first use in combination with memantine. Data for one patient were taken in a laboratory while for the other, a clinical environment; the surgeries were performed at different medical centers by different surgeons. We also demonstrate a novel extension of the NAFX (see Methods) to estimate post-therapy improvement in measured visual acuity.
| | | Materials and Methods | METHODS
(Case 1)
Recording
We measured horizontal, vertical, and torsional rotations of each eye and head position using a magnetic-field search coil.(2) The system had a linear range greater than ±20° with a sensitivity of 0.1°, and crosstalk less than 2.5%. Monocular primary-position calibration allowed accurate position information and documentation of small tropias and phorias hidden by the nystagmus. The total system bandwidth was 0-100 Hz; the data were digitized at 500 Hz with 16-bit resolution. We measured fixation (at far, near, and eccentric gaze angles), saccades, smooth pursuit, and vestibulo-ocular reflex, as previously described.(2)
Protocol
Written consent was obtained before eye-movement testing, eye muscle surgery, and treatment with memantine as part of our approved IRB protocol. All test procedures were carefully explained to the subject and reinforced with verbal commands during the trials. Subjects were seated in a chair with headrest and either a bite board or a chin stabilizer in a dimly lit room, far enough from an arc of red LEDs to prevent convergence effects (>5 feet). The LEDs subtended less than 0.1° of visual angle. These easy visual tasks avoid stress that might affect the patient’s eye movements.
Analysis
We measured average values of peak-to-peak velocity for the same fixation intervals used to predict visual acuity and median eye speed of each nystagmus component based on 10-second (5000 points) segments. We also compared pre- and post-therapy velocities of saccades and fast phases of optokinetic and vestibular nystagmus.
The eXpanded Nystagmus Acuity Function (NAFX)
The NAFX is an unbiased mathematical function based on eye-movement data that characterizes the foveation profile/quality of nystagmus waveforms.(9, 10) Initially developed to evaluate patients with INS and fusion maldevelopment nystagmus syndrome (FMNS),(4) the NAFX is a unique, direct measure of the motor effects of therapies aimed at altering nystagmus, The NAFX is linearly correlated to potential, best-corrected Snellen (decimal) visual acuity, predicts potential acuity improvement, quantifies the portion of visual acuity loss attributable to afferent deficits,(13) and, as demonstrated in this study, predicts post-therapeutic improvements in measured visual acuities. A newly automated version of the NAFX software (written for MATLAB, The Mathworks, Natick, MA) may be downloaded from www.omlab.org (“Software and OMS Models” page). Radial data,(14) derived from the horizontal and vertical data, was used to calculate the NAFX for patient 1’s multiplanar nystagmus. This biplanar NAFX, from fixating-eye data segments during binocular viewing, provided pre- and post-therapy comparisons; averaged values were used to assess potential, and estimate measured, acuity improvements.
Age-appropriate NAFX vs. acuity lines based on the fiftieth percentile of distribution of measured visual acuity in the population (data from FW Weymouth plotted by G Westheimer)(15) provide accurate comparisons of potential patient acuities and acuity improvements, presuming no afferent deficits. A line of the same slope (dashed “Estimated Improvement” line) through the pre-tenotomy measured acuity data point, allows estimation of the patient’s increase in measured acuity subsequent to therapy (i.e., presuming no changes in the afferent system). The line’s endpoint (at NAFX = 1.0) predicts the maximal measured acuity possible for the patient if the therapy abolished all nystagmus.
(Case 2)
Digitized Videotapes
The three analog videotapes of the second patient’s eye movements were digitized using a Sony DV camcorder and QuickTime (Apple Computer, Inc. Cupertino, CA) movies of the most relevant portions of the video were created in uncompressed AVI format at 30 frames/sec. These files were analyzed frame-by-frame in MATLAB 7, using custom-written software that quantified the motion of the centroid of the pupil. The resulting waveform’s frequency content was examined using a fast Fourier transform (FFT), and low-pass filtered at twice the largest major component, or 10 Hz. This filtered signal was graphically analyzed to determine peak-to-peak amplitude of the nystagmus.
Surgery (Cases 1 and 2)
The tenotomy procedure consists of detaching the muscle at the enthesial, or insertion, end of the tendon and reattaching it in the same place by means of a double-armed suture.(8, 10, 16) In this paper, when referring to the surgical procedures performed, the word “tenotomy” includes the reattachment of the tendons.
| | | Results | CASE REPORTS
Case 1. A 50 year-old man with MS for ~10 years, presented with the chief complaint of, “there is shaking all the time.” His oscillopsia had been slowly progressing over the prior 8-9 years due to APN; both were refractive to medical treatment. Prior to the diagnosis of MS, he had no history of childhood strabismus, nystagmus, acute optic neuritis, or acute visual loss. For the prior year he had intermittently closed his right eye and recently admitted to diplopia. On initial, independent examinations by two physicians on different dates, visual acuities with his distance correction were 20/200 and 20/160 OD (J16 at near), 20/100+ and 20/160 OS (J16 at near), and 20/100 and 20/150 OU (binocularly). Low contrast visual acuity with the 10% Sloane Low Contrast Chart was worse than 20/200 OD and 20/200 OS. He identified 9.5 of 13 Ishihara color plates OD and 12 of 13 OS. A right relative afferent pupillary defect and APN with horizontal, vertical and torsional components (similar amplitude in each eye, and less in downgaze) were noted on initial exam. He had a 25-30D exotropia by Krimsky test and a bilateral internuclear ophthalmoplegia. Dilated fundoscopy showed questionable mild pallor of both optic discs, suggesting chronic sub clinical optic nerve demyelination, with no other findings. Visual fields, using the Humphrey 81-3 full-field screening test, showed no abnormalities with either eye but fixation was impaired by the APN.
We initially planned a two-stage surgical approach similar to the treatment of multiplanar nystagmus in the achiasmatic Belgian sheepdog.(8) A four-muscle tenotomy of the horizontal rectus muscles OU combined with recessions of both lateral rectus muscles (to correct his exotropia) was to be followed, in 4-6 months, by vertical rectus-muscle tenotomies OU (plus possible inferior rectus-muscle recessions) to damp the vertical component. The first stage (performed by RL Tomsak) went without complications. Post-operatively, the patient had 10D of esotropia and reduction of APN. His corrected visual acuity, measured by the same two physicians on different dates, improved to 20/60- and 20/100 OD, 20/60- and 20/80 OS, and 20/100 OU. Sloane low contrast acuity was 20/125 OD and 20/160 OS. Near vision without correction was J1 at about 8”. He preferred a 15D BO prism OD. The second stage (vertical tenotomy procedure) was deferred when memantine became available. After discussion of potential benefits and risks, and published studies(12) with the patient and his personal physician, a trial of memantine was begun in an attempt to further damp the APN.
Memantine was increased over six weeks to a daily dose of 40 mg (twice the dose used for Alzheimer’s disease) without complication. After 6 weeks, at 40 mg/day (given in 4 10-mg doses), the best-corrected visual acuity was 20/50 OD, 20/50 OS, and 20/50 OU. Sloane low contrast acuity was 20/80 OD and 20/80 OS.
CASE 1 - EYE MOVEMENT RESULTS
Pre-surgery APN
Prior to surgery, the waveforms in each plane were dual jerk(17) with a 0.67 Hz jerk component (to the left, down, and counter-clockwise), caused by the saccadic pulses of the patient’s internuclear ophthalmoplegia (sometimes mistakenly labeled “abduction nystagmus”), and a 3.5 - 4 Hz pendular component. The latter was phase-locked in both eyes in the horizontal and vertical planes (but 90° out of phase between the horizontal and vertical planes, resulting in counter-clockwise elliptical motion). The two eyes were 90° out of phase in the torsional plane, as can be seen using the vertical grid lines in Figure 1. The horizontal and vertical nystagmus scanpaths plus the torsional conjugacy plot of Figure 2 illustrate the resulting eye motion. Both Figures show that, although phase locked, the APN radial amplitudes differed in each eye (typically ~1.8°pp in the right eye and ~2.2°pp in the left). The abducting saccadic pulses of internuclear ophthalmoplegia where superimposed on the APN and mimicked a dual jerk nystagmus waveform. Also evident was the characteristic dissociation between abducting and adducting saccades. Figure 2 shows complex elliptical APN trajectories in both eyes and conjugate, diagonal saccadic pulses (straight lines from lower left to upper right). The elliptical APN trajectories in the torsional conjugacy plot indicate a 90° phase shift between the eyes and the straight lines from lower left to upper right, conjugate saccadic pulses.
Post-surgery APN
The first surgical phase reduced the horizontal APN markedly. Figure 3 (data taken 1 month post horizontal surgery) shows the reduced peak-to-peak horizontal APN velocities in either fixating eye. Post-surgery slow-phase velocities decreased by 38-77.5% in the right eye and 50% in the left eye; nystagmus amplitudes also decreased by 50%. Figure 4 (nystagmus scanpaths) demonstrates both the APN damping in both eyes (regardless of which eye was fixating) and correction of the exotropia. Note that the vertical APN was also damped. Direct comparison of the peak-to-peak horizontal, vertical, and torsional APN velocities revealed vertical and some torsional damping.
Post-surgery saccades, pursuit, OKR, and VOR
There were no substantial post-operative changes in saccadic dynamics, smooth-pursuit, optokinetic, or vestibular eye movements.
Post-surgery, post-memantine APN
Instead of proceeding to the second surgical stage (on the four vertical rectus muscles), we studied the effects of memantine on the residual nystagmus 7 months post surgery. Figure 5 demonstrates the intercycle variability of the APN in all planes at all stages of therapy; the transiently high saccadic velocities are from superimposed saccadic pulses and should be ignored. In this segment, memantine had the greatest effect on the residual vertical component (that had been slightly damped by the prior horizontal surgery) and less effect on the residual horizontal and torsional APN components. Figure 6 shows the overall effects of horizontal surgery and memantine on the median speeds (in each plane and radially) of the nystagmus. Horizontal surgery mainly reduced horizontal speeds with some reduction in vertical and torsional speeds. Memantine reduced speeds in all planes. The surgery reduced APN to 58% of its pre-surgery value and memantine + surgery further reduced it to 17.8%. This 82.2% overall improvement corresponded to the patient’s estimate of a 75% decrease in his oscillopsia.
Post-surgery, post-memantine NAFX and visual acuity
Additional evaluations of the direct effects of both therapies on APN were made using the NAFX. The Table contains the NAFX, predicted best-corrected visual acuities (presuming neither afferent deficits nor oscillopsia), measured visual acuities (reflecting the APN, afferent deficits, and oscillopsia), and the changes in APN amplitude (normalized to a pre-surgery value of 100%). As expected, the NAFX-predicted potential acuities far exceeded the measured values, due to the patient’s afferent deficits (and possibly, oscillopsia). The waveform improvements caused by both therapies resulted in improved measured visual acuities.
The dashed “Estimated Improvement” line in Figure 7 predicts a maximum measured acuity of ~0.72 = 20/30+ for an NAFX = 1.0 (e.g., if therapy abolished all nystagmus). After the horizontal surgery stage, NAFX-estimation of improved potential and measured acuity was 0.197; the measured acuity improvement was 0.137. After surgery + memantine, NAFX-estimation of improved potential and measured acuity was 0.071; the measured acuity improvement was 0.15.
Case 2. The patient was a 39 year-old female with MS. Her major complaint was reduced vision and oscillopsia inn her right eye. She was initially taking interferon beta-1a, amitriptyline, gabapentin (100 bid), and modafinil. She exhibited a clinically evident pendular nystagmus in her right eye but none in her left eye (documented by videotape). Her vision was 20/100 in the affected right eye and 20/20 in the normal left eye. She underwent the above tenotomy and reattachment procedure of the horizontal rectus muscles of her right eye (performed by DC Bienfang). Six weeks after surgery, she was examined while taking gabapentin (900/day) for foot problems. Her right-eye nystagmus was substantially reduced (confirmed by video tape) and her visual acuity was 20/70 OD. She discontinued the gabapentin and was examined 5 months after surgery when two independent ophthalmologists measured her visual acuity as 20/60 and 20/50 OD. A third video tape recording of her right eye documented the residual nystagmus. She had no complications (e.g., diplopia) from the tenotomy procedure. Her right eye had much better vision and she reported that the oscillopsia was “much reduced.”
CASE 2 - EYE MOVEMENT RESULTS
Analog videotape data were available to supplement the clinical observations for this much simpler APN, being predominantly horizontal and limited to the right eye. The digitized video recordings revealed that the 4.5 Hz APN of the right eye was reduced by 66% by the two-muscle tenotomy of the horizontal rectus muscles of that eye. Figure 8 shows segments of the pre- and post-tenotomy (with and without gabapentin) nystagmus of Case 2. The post-tenotomy procedure nystagmus was not altered by gabapentin. There was a sub-clinical, horizontal APN in the left eye that was too small to analyze from the digitized videotape data.
| |
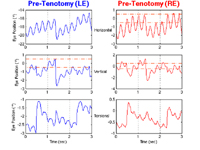
Figure 1.
Pre-surgery horizontal, vertical, and torsional nystagmus waveforms from Case 1 of the left (LE) and right (RE) eyes during RE fixation with the LE exotropic. Dash-dotted lines indicate foveal extent in this and subsequent eye-position plots. Vertical grid lines show conjugacy in the horizontal and vertical planes, 90° phase shifts between the horizontal and vertical components of each eye, and a 90° torsional phase shift between the eyes. In all Figures, positive values indicate rightward, upward, or clockwise motion from the patient’s point of view.
|
|
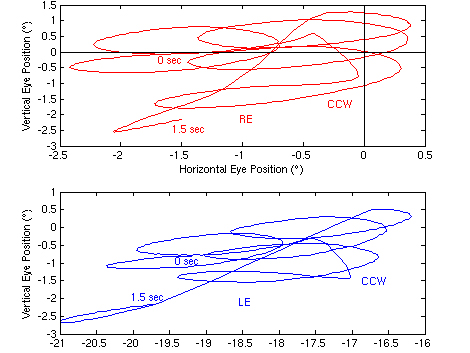
Figure 2.
Pre-surgery nystagmus scanpaths (horizontal vs. vertical) from Case 1 of the right (RE) and left (LE) eyes (top and middle traces, respectively), and torsional conjugacy plot (RE vs. LE, bottom trace). These plots are from the first 1.5 seconds of data shown in Figure 1 with the starting and ending time points shown; the RE is fixating and the LE is exotropic. The elliptical scanpaths moved in a counter clockwise (CCW) manner and the elliptical conjugacy plot indicates a 90° torsional APN phase difference between the eyes. In both scanpaths, the straight portions (from upper right to lower left) are the conjugate, diagonal saccadic pulses; in the conjugacy plot (from lower left to upper right), they are the conjugate, clockwise saccadic pulses.
|
|
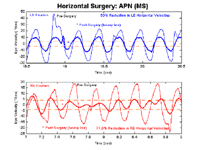
Figure 3.
Pre- and post-surgery plots of the horizontal components of the APN from Case 1 of the left (LE) and right (RE) eyes during LE fixation (top) and RE fixation (bottom). There was significant damping of both eyes as a result of the tenotomy surgery. The different scales reflect the amplitude difference in the two eyes. MS is multiple sclerosis. In this and Figure 5, dash-dotted lines indicate the ±4°/sec limits of good visual acuity.
|
|

Figure 4.
Pre- and post-surgery scanpaths (horizontal vs. vertical) from Case 1 of the right (RE) and left (LE) eyes during fixation by either. The horizontal component of the APN was damped and the exotropia eliminated (in this segment, a small exotropia remained). There was also damping of the vertical component of the APN as a result of the horizontal rectus tenotomy and strabismus surgery. BE Viewing – both eyes viewing (open).
|
|
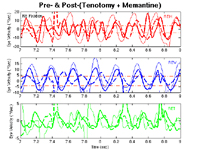
Figure 5.
Pre- and post-surgery, and post-(surgery + memantine) plots from Case 1 of the horizontal (REH), vertical (REV), and torsional (RET) components of the APN of the right (RE) eye during RE fixation. There was some damping of the vertical component as a result of the horizontal tenotomy surgery and additional damping of the horizontal, and significant additional damping of the vertical, APN as a result of the memantine. Heavy solid lines are post-surgery plots; heavy dashed lines are post-(surgery + memantine) plots. Asterisks (*) in the vertical traces indicate the occurrence of saccadic pulses with variable high velocity components in all planes; they should be ignored.
|
|

Figure 6.
Eye speeds vs. recording sessions from Case 1 for the fixating, right eye. Radial speeds calculated from horizontal and vertical speeds.
|
|
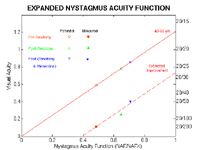
Figure 7.
The expanded nystagmus acuity function (NAFX) vs. potential visual acuity (solid line) for the 40 to 60 year old age range with the potential and measured acuity values for Case 1 shown pre-surgery, post-surgery, and post-(surgery + memantine). The dashed line is the NAFX-estimate of the increases in this patient’s measured acuity due to nystagmus waveform improvement. The open symbols are the patient’s average NAFX values plotted on the potential acuity line and the solid symbols are these same NAFX values plotted at their respective average measured acuities. The differences at each stage of therapy between the potential and measured acuity values are due to the patient’s afferent deficit.
|
|
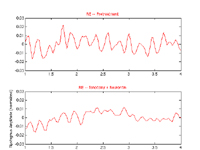
Figure 8.
Segments of the right-eye (RE) horizontal nystagmus reconstructed from the digitized videotape records from Case 2. Shown are pre-treatment, post-(tenotomy + gabapentin), and post-tenotomy records normalized to the distance between the canthi. Solid lines are the low-pass filtered data points.
|
|
| Discussion | DISCUSSION
Current evidence suggests that APN arises within the gaze-holding “neural integrator” for eye movements, which depends on circuits between the brainstem and cerebellum.(1, 18) Tenotomy presumably damps nystagmus by altering ocular proprioceptors which lie close to the insertion point of the extraocular muscles.(16, 19-22) Disrupting such proprioceptive pathways may change the overall “gain” of a feedback system that might be similar to the gamma-efferent system for voluntary muscle.(8, 10) Thus, the ocular motor system’s periphery is altered with respect to the effects of the APN motor signal on the actual oscillation of the eyes (Cases 1 and 2). Memantine acts centrally to reduce the APN motor signal in the brainstem (Case 1). Because of the different sites of action, these two interventions should act independently and synergistically on APN. In Case 1, each therapy reduced APN and in combination, the effects were multiplicative. In Case 2, two-muscle tenotomy and reattachment on the affected eye damped its APN.
The NAFX is an ideal measure of nystagmus therapies because it combines a direct-outcome measure of the therapy (e.g., nystagmus waveform improvement) with a predictive measure of the medical goals of improved visual function (e.g., visual acuity). For therapies designed to correct afferent visual dysfunction (e.g., gene therapy for RPE65-deficient canines(23)), the electroretinogram, pupillary light reflex, and visual evoked potential are the most direct measures. Each is also predictive of visual acuity, which is determined upstream, and requires higher cortical function. The best measure of both cosmesis (how the nystagmus looks) and oscillopsia is the direct measure of APN amplitude (or velocity); this study includes all three types of measures.
In Case 1, four-muscle tenotomy procedure (including two-muscle recession) damped the horizontal component of APN, increasing the NAFX, improving visual acuity, and reducing oscillopsia. We have no explanation for the additional, unexpected, and therapeutically fortuitous result of damping the vertical component. In an achiasmatic Belgian sheepdog with congenital see-saw nystagmus, the first stage of treatment was also horizontal muscle surgery. Any effects on the vertical components of the dog’s nystagmus were masked by the see-saw movements.(8) Cross-plane damping suggests that motion in each plane of the ocular motor plant (the extraocular muscles, supporting tissues, and globe) may not be totally independent and that, even at this peripheral level, changing the plant gain in one plane might affect the gains in other planes. It is possible that realignment of the eyes—converting from an exotropia to an esotropia—affected APN, although single vision was not restored. There are no data in the literature that suggest, nor have we ever observed, damping of nystagmus subsequent to recession of a muscle (or both antagonist lateral rectus muscles) to realign a strabismic eye. Thus, we do not ascribe the APN damping to the strabismus portion of the surgery. The tenotomy procedure’s effectiveness in treating both APN and INS, supports its hypothetical, proprioceptive mechanism of action(8, 10) that results in a small-signal (not the large bursts generating saccades) gain reduction of the plant, as do the unaffected saccades and fast phases of the OKR and VOR. Also consistent with this interpretation is the greater reduction of the horizontal APN, corresponding to the muscles that were operated. Current, linear models of the plant require that gain reductions affect all eye movements, not just APN. The possibilities of proprioceptive small-signal gain-control and interplane crosstalk complicate this simple conception and are inconsistent with current linear models of the plant.
In Case 2, the two-muscle tenotomy procedure of the affected eye’s horizontal rectus muscles was sufficient to damp that eye’s APN—the first demonstration that for uniocular nystagmus, only the affected eye requires a tenotomy procedure. It is not known if the procedure’s effectiveness is limited to that eye, or if nystagmus in the other eye would also have been damped, if present. Also unknown is the effect of tenotomy and reattachment on only one of the two involved muscles. We are currently trying to determine this from strabismus surgeries on patients with INS and FMNS.
Possible Mechanisms for Memantine (Case 1)
Memantine, following horizontal surgery, reduced all components of the patient’s residual APN and oscillopsia and further improved his vision. Memantine is a low-to-moderate affinity uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist which reduces glutamatergic excitotoxicity, among other effects.(24) Its exact mechanism of action on APN is unknown, but presumably involves some effect on glutamate. Pharmacological inactivation studies in monkeys(25) identified glutamate to be a neurotransmitter in the medial vestibular nucleus—nucleus prepositus hypoglossi complex in the medulla, essential for gaze-holding (neural integrator) function, especially in the horizontal plane. Gabapentin may also effect APN via a glutamate mechanism, since more purely GABAergic drugs are ineffective.(3) Because memantine suppressed all components of APN, not just the horizontal, it seems likely that its action was independent of the prior surgery; this independence may be important for patients in whom one therapeutic measure proves insufficient.
The NAFX, APN, Oscillopsia, and Measured Acuity (Case 1)
The NAFX provided a direct, and measured visual acuity an indirect, measure of the therapeutic effects of both therapies on the APN. The slightly different measured acuity values (averaged from independent examinations on two days) may reflect changes in the afferent deficit (common in MS) or different testing conditions and examiners. The NAFX estimated 33.6% improvement in potential acuity as a result of surgery and additional 9% improvement for surgery + memantine. The pre- and post-therapy measured acuities were lower than the potential acuities because of the patient’s afferent deficit. The NAFX estimated increases in measured acuity of 174% for the surgery and an additional 28% for the surgery + memantine therapies; the actual measured increases were 121% and 60%, respectively, following the slope of the estimation line in Figure 7. Because that line also showed the maximum achievable acuity (if therapy eliminated all nystagmus) to be ~0.72 = ~20/30+, we conclude that the afferent deficit alone in this patient caused a decrease in acuity of ~0.5 (i.e., visual acuity decreased from a potential of ~20/16- to ~20/30+).
Dynamic visual acuity, measured under conditions of a moving target, is less than static acuity because of retinal image motion and possibly associated perceived motion (oscillopsia).(26-28) In APN, oscillopsia may have detrimental effects on acuity in a patient similar to actual target motion in a normal, reducing measured acuity. Peak-to-peak amplitude is a poor predictor of acuity in INS. Unlike INS, the APN waveform (having no extended foveation periods) remains relatively unchanged and amplitude variations could affect acuity more directly.
We wondered if the oscillopsia of APN would result in a lower acuity than in INS without oscillopsia. The percent change in APN amplitude/speed was greater than in the NAFX (42% and 69% for surgery and surgery plus memantine respectively, see Figure 6). This implies that the significant oscillopsia reduction caused by the APN damping was not the major determinant of acuity improvement in this patient (i.e., measured acuity was not higher than predicted by the dashed NAFX line). Thus, although debilitating, oscillopsia did not appear to have a significant role in visual acuity reduction. Visual acuity improvement was determined primarily by nystagmus foveation improvement, consistent with NAFX estimations.
The afferent deficits could have transiently changed for reasons unrelated to the therapies or, memantine may have improved vision. The high NAFX values indicated that the portion of the total acuity deficit due to the APN was minimal. The largest contribution to the patient’s low pre-therapy acuity (the difference between measured and potential acuity shown in Figure 7) appeared to be from the afferent deficit. Because the dashed NAFX line correctly estimated the improvements in measured acuity, the latter were due mainly to nystagmus waveform improvements. Pre- and post-memantine data do suggest the possibility of some afferent-system improvement (i.e., the measured acuity improvement slightly exceeded the estimation).
Other Observations on Dual-mode Therapy (Case 1)
We applied a dual-mode therapeutic approach (surgical and pharmacological) and demonstrated that each therapy, acting with a different mechanism and anatomical site, reduced the APN and increased visual acuity. Adopting a simple linear treatment model, the therapeutic effects of surgery and memantine at their respective sites are independent and the total effect, multiplicative. From Figure 6, the effect of the memantine on the source of the APN was calculated to be 17.8/58 = 0.31 (i.e., APN was reduced by 69%). Thus, had the memantine been the first therapy instead of tenotomy and reattachment, it may not have completely suppressed the nystagmus. A second-stage tenotomy procedure of the four vertical rectus muscles should further reduce the vertical component of the APN. However, it would not make as much of an improvement post-memantine as pre-memantine. Thus, a second surgical stage for patient 1 is a clinically viable option only if memantine is discontinued or if the vertical APN component becomes more evident and symptomatic.
In summary, the combination of a peripheral-surgical and a central-pharmacological therapy resulted in a greater reduction of both the APN and oscillopsia symptoms than either one alone. Our results suggest that surgical and pharmacological therapies may be employed separately or in conjunction, and in either order. The choice of therapy for others with APN will depend on the severity of symptoms, their goals and expectations, and the idiosyncratic effects of the therapies. Tenotomy and reattachment is a simple, low-risk, outpatient procedure whose benefits are permanent, with no side effects. Memantine, although non-surgical, requires continuous use and may have undesirable side effects. If the therapeutic goal is to minimize symptoms and maximize visual acuity, a dual-mode approach seems most promising and deserves further study.
Future Applications
The effectiveness of the tenotomy procedure in APN expands its therapeutic use to patients whose symptoms seriously impair their quality of life. Eye-muscle surgery also provides an opportunity to realign the eyes in patients with acquired strabismus. The nystagmus component of eye-muscle surgery (i.e., tenotomy) affects the oscillation whether or not it is combined with a strabismus component (i.e., repositioning of the cut tendon) because the enthesial tendon detachment is what all these procedures have in common. Although side effects of memantine are usually mild, patients with neurological disease such as MS will require careful monitoring. A formal FDA-approved trial of memantine versus gabapentin is underway to compare the two agents as treatment for acquired forms of nystagmus.
| | | Acknowledgements | This work was supported in part by: the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (lfd,rjl); NIH Grant EY06717 (rjl); Evenor Armington Fund (rjl) and NIH Training Grant EY07157 (jbj).
Presented in part at the 2004 ARVO and 2005 NANOS meetings.
Reprint requests and correspondence to:
L.F. Dell’Osso, Ph.D.
Daroff-Dell’Osso Ocular Motility Laboratory
Louis Stokes Cleveland Veterans Affairs Medical Center
10701 East Boulevard
Cleveland, OH 44106 USA
Telephone: (216) 421-3224—FAX: (216) 231-3461
E-mail: lfd@cwru.edu—Web Site: omlab.org
We are grateful for technical assistance from Yanning Han and Arun Kumar and for Dr. Mary R. Rensel, who referred patient 1 to our laboratory to evaluate him for possible tenotomy surgery.
| | | References | REFERENCES
1. Leigh RJ, Zee DS. The Neurology of Eye Movements, Edition 3 (Contemporary Neurology Series). New York: Oxford University Press, 1999:436-439.
2. Averbuch-Heller L, Tusa RJ, Fuhry L, et al. A double-blind controlled study of gabapentin and baclofen as treatment for acquired nystagmus. Ann Neurol 1997;41:818-825.
3. Bandini F, Costello E, Mazella L, Mancardi GL, Solaro C. Gabapentin but not vigapentin is effective in the treatment of acquired nystagmus in multiple sclerosis: how valid is the GABAergic hypothesis? J Neurol Neurosurg Psychiatr 2001;71:107-110.
4. CEMAS_Working_Group. A National Eye Institute Sponsored Workshop and Publication on The Classification of Eye Movement Abnormalities and Strabismus (CEMAS). In The National Eye Institute Publications (www.nei.nih.gov). Bethesda, MD: National Institutes of Health, National Eye Institute, 2001.
5. Roberts EL, Saunders RA, Wilson ME. Surgery for vertical head position in null point nystagmus. J Pediatr Ophthalmol Strabismus 1996;33:219-224.
6. Jain S, Proudlock F, Constantinescu CS, Gottlob I. Combined pharmacologic and surgical approach to acquired nystagmus due to multple sclerosis. Am J Ophthalmol 2002;134:780-782.
7. Depalo C, Hertle RW, Yang D. Eight muscle surgical treatment in a patient with acquired nystagmus and strabismus. Binoc Vis Strab 2003;18:151-158.
8. Dell'Osso LF, Hertle RW, Williams RW, Jacobs JB. A new surgery for congenital nystagmus: effects of tenotomy on an achiasmatic canine and the role of extraocular proprioception. J AAPOS 1999;3:166-182.
9. Hertle RW, Dell'Osso LF, FitzGibbon EJ, Thompson D, Yang D, Mellow SD. Horizontal rectus muscle tenotomy in patients with infantile nystagmus syndrome: a pilot study. JAAPOS 2004;8:539-548.
10. Hertle RW, Dell'Osso LF, FitzGibbon EJ, Thompson D, Yang D, Mellow SD. Horizontal rectus tenotomy in patients with congenital nystagmus. Results in 10 adults. Ophthalmology 2003;110:2097-2105.
11. Dell'Osso LF. Extraocular muscle tenotomy, dissection, and suture: A hypothetical therapy for congenital nystagmus. J Pediatr Ophthalmol Strab 1998;35:232-233.
12. Starck M, Albrecht H, Pollmann W, Straube A, Dieterich M. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol 1997;244:9-16.
13. Dell'Osso LF, Jacobs JB. An expanded nystagmus acuity function: intra- and intersubject prediction of best-corrected visual acuity. Doc Ophthalmol 2002;104:249-276.
14. Averbuch-Heller L, Dell'Osso LF, Leigh RJ, Jacobs JB, Stahl JS. The torsional component of 'horizontal' congenital nystagmus. J Neuro-Ophthalmol 2002;22:22-32.
15. Westheimer G. Visual acuity. In: Kaufman PL, Alm A, editors. Adler's physiology of the eye. Clinical applicatioin. St. Louis: Mosby, 2003:453-469.
16. Hertle RW, Chan C, Galita DA, Maybodi M, Crawford MA. Neuroanatomy of the extraocular muscle tendon enthesis In macaque, normal human and patients with congenital nystagmus. J AAPOS 2002;6:319-327.
17. Dell'Osso LF, Daroff RB. Congenital nystagmus waveforms and foveation strategy. Doc Ophthalmol 1975;39:155-182.
18. Das VE, Oruganti P, Kramer PD, Leigh RJ. Experimental tests of a neural-network model for ocular oscillations caused by disease of central myelin. Exp Brain Res 2000;133:189-197.
19. Büttner-Ennever JA, Horn AKE, Graf W, Ugolini G. Modern concepts of brainstem anatomy. From extraocular motoneurons to proprioceptive pathways. In: Kaminski HJ, Leigh RJ, editors. Neurobiology of Eye Movements. From Molecules to Behavior—Ann NY Acad Sci 956. New York: NYAS, 2002:75-84.
20. Konakci KZ, Streicher J, Hoetzenecker W, Blumer MJ, Lukas JR, Blumer R. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest Ophthalmol Vis Sci 2005;46:155-165.
21. Jaggi GP, Laeng HR, Müntener M, Killer HE. The anatomy of the muscle insertion (scleromuscular junction) of the lateral and medial rectus muscle in humans. Invest Ophthalmol Vis Sci 2005;46:2258-2263.
22. Eberhorn AC, Horn AKE, Fischer P, Büttner-Ennever JA. Proprioception and pallisade endinge in extraocular eye muscles. In: Ramat S, Straumann D, editors. Clinical and Basic Oculomotor Research. In Honor of David S. Zee—Ann NY Acad Sci 1039. New York: NYAS, 2005:1-8.
23. Jacobs JB, Dell'Osso LF, Hertle RW, Bennett J, Acland GM. Gene therapy to abolish congenital nystagmus in RPE65-deficient canines: Annual Meeting Abstract and Program Planner [on CD-ROM or accessed at www.arvo.org], 2003:ARVO Abstr 4249.
24. Mobius HJ, Stoffler A, Graham SM. Memantine hydrochloride: pharmacological and clinical profile. Drugs of Today 2005;40:685-695.
25. Arnold DB, Robinson DA, Leigh RJ. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vision Res 1999;39:4286-4295.
26. Westheimer G, McKee SD. Visual acuity in the presence of retinal-image motion. J Opt Soc Am 1975;65:847-850.
27. Currie DC, Bedell HE. Visual acuity in normal subjects with simulated nystagmus. Optom Vis Sci 1989;66:145.
28. Currie DC, Bedell HE, Song S. Visual acuity for optotypes with image motions simulating congenital nystagmus. Clin Vis Sci 1993;8:73-84.
| |
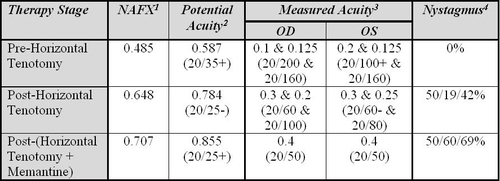
Table. Pre- and Post-Therapy NAFX and Visual Acuity Values for Case 1
1 Biplanar (horizontal and vertical) expanded nystagmus acuity function (NAFX)
2 Best-corrected for 40 to 60 year-old adults who have neither afferent deficits nor oscillopsia
3 This patient who has both afferent deficits and oscillopsia
4 Percent decreases normalized to the pre-surgery nystagmus (horizontal/vertical/radial)
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in