|
|
 |
 |
 |
 |
|
|
Characterization of an Outbreak of Acute Hemorrhagic Conjunctivitis in Mexico, 2003
Digital Journal of Ophthalmology 2005
Volume 11, Number 1
January 9, 2005
|
Printer Friendly
|


 Gerardo Chowell
Gerardo Chowell | Cornell University Carlos Castillo-Chavez | Arizona State University Porfirio Diaz-Duenas | Instituto Mexicano del Segurio Social
|
|
|
| Abstract | Objective
To estimate the number of secondary cases generated by a primary case of acute hemorrhagic conjunctivitis using epidemic curve data and report on the relative frequency of the symptoms presented, the spatial, the age-specific incidence, and the time from onset of symptoms to diagnosis distributions.
Methods
We analyzed 886 cases of acute hemorrhagic conjunctivitis for which clinical symptoms were fully recorded by public health clinics of the Mexican Institute of Public Health (IMSS) in the state of Colima, Mexico during the period of September - November 2003.
Results
We estimate the number of secondary cases generated by a primary infectious case of acute hemorrhagic conjunctivitis (AHC) to be approximately 4. Most of the AHC cases occurred in the coastal region of the state of Colima, Mexico (93.6%). The most common reported symptom was excessive tearing of the eye or epiphora (92.9%) followed by conjunctival hyperemia (65.8%), photophobia (54.1%), subconjunctival hemorrhage (48.1%), eye pain (47.5%), and palpebral chemosis (36.4%). Less common symptoms were conjunctival edema (24.2%), blurry vision (10.4%) and fever (9.0%). ). The maximum likelihood estimates of the mean and variance for the time of onset to diagnosis were 1.7 days (95% CI 1.59-1.82) and 2.9 days, respectively.
Conclusion
Our estimate of the basic reproductive number (estimated to be approximately 4 secondary cases per primary infectious case) highlights the power of AHC to spread. Therefore, its control should be taken seriously as AHC outbreaks can have important economic consequences. Since there is no specific treatment for AHC, rapid control of AHC relies on contact tracing followed by education of symptomatic infectious individuals.
Keywords
Acute Hemorrhagic Conjunctivitis, pink eye, basic reproductive number, time to diagnosis. | | | Introduction | Acute hemorrhagic conjunctivitis (AHC) is a highly communicable disease typical of tropical, coastal cities. AHC outbreaks usually last 1-2 months and secondary attack rates within households are greater than 50% [1]. A susceptible individual exposed to the virus enters a short incubation period (1-2 days) followed by the rapid onset of painful, swollen, red eyes, lacrimation, foreign-body sensation, and subconjunctival hemorrhaging in many cases [2]. Transmission occurs primarily via person-to-person contact or contact with contaminated objects (i.e towels). Symptoms persist usually for 3-7 days [1].
There have been identified different viruses as the causing agent of AHC including enterovirus 70, coxsakievirus A24 variant (CA24v) and adenovirus 11 [3,4].
An outbreak of AHC recently occurred in Mexico during the period September-December 2003. 67,513 cases had been reported as of September 26th, 2003 [5]. Among the most affected Mexican states by AHC were Campeche, Quintana Roo and Tabasco [5]. For the state of Jalisco, the incidence rate was of 141.8 cases per 100,000 inhabitants [6] and the causal agent was isolated and identified as an Adenovirus (L. Salazar-Montes, Secretary of Public Health in Jalisco, Mex., email communication).
| | | Materials and Methods | An outbreak of Acute Hemorrhagic Conjunctivitis (AHC) developed in the state of Colima, Mexico during the period of September-November, 2003. The state of Colima is located on the pacific coast, with a tropical climate, a mean temperature of 23-26 ºC, and an approximate population of 488,028 [7]. Here, we analyzed 886 cases of AHC for which clinical symptoms were fully recorded by public health clinics of the Mexican Institute of Public Health (IMSS) which currently provides service to approximately 60% of the population in the state of Colima, Mexico.
We estimate the basic reproductive number (R0) of the disease from the initial exponential growth phase of the outbreak. We report on the relative frequency of the symptoms presented, the spatial, the age-specific incidence, and the time from onset of symptoms to diagnosis distributions.
The course of the outbreak was characterized by an initial exponential growth phase from approximately September 3rd to September 27th when the epidemic reached its peak with 101 cases on September 29th followed by a fast decline in the daily number of cases until November 11th. The course of the outbreak presented in Figure 1 does not intend to represent the “true” epidemic but rather it is a good representative sample of the time course of the epidemic as the data reported here was obtained from the Mexican Institute of Public Health (IMSS) that provides service to approximately 60% of the population of the state of Colima, Mexico. Moreover, underreporting is common during AHC outbreaks (it is estimated that for every reported case there are 4 cases that are not reported [6]).
| | | Results | The basic reproductive number (R0)
The basic reproductive number (R0) quantifies the power of a disease to spread and can be defined as the number of secondary cases generated by a primary infectious case when this is introduced into a fully susceptible population. However, once an epidemic is underway, the reproductive number (Rt) decreases over time (t) because the number of susceptible individuals decreases, behavioral changes in the population occur and public health interventions are implemented. We estimate the basic reproductive number (R0) from the initial exponential growth phase of the cumulative number of cases (Figure 1). The estimated intrinsic growth rate r = 0.34. The basic reproductive number can be estimated using the formula [9]:
R0 = 1 + r2 t1 t2 + r (t1 + t2)
where t1 is the incubation period and t2 is the infectious period of the disease. The above formula assumes that infected individuals are not infectious during their incubation period (t1). Hence, we can use this formula to estimate the R0 of AHC as infected individuals become infectious once they become symptomatic. Moreover, the incubation period (t1) for AHC is small and ranges between 1 and 2 days [2] while the infectious period (t2) is estimated to range from 3 to 7 days with a mean of 5 days [1]. Hence, we estimate a reproductive number for AHC ranging between 2.7 and 5.7 with a mean of 4.1 (t1 = 1.5 days, t2 = 5 days). The estimated basic reproductive number is consistent with the rapid initial increase in the number of cases for approximately four weeks (this has been observed in other AHC outbreaks [1]). After the first four weeks of the outbreak, the number of cases decreases rapidly until the outbreak is over (Figure 1). This behavior can be attributed to the rapid depletion of susceptible close contacts (required for transmission) as infectious cases tend to infect their relatives when they are at home under isolation and hence their potential to infect individuals outside their families is greatly reduced. In fact, the secondary attack rates within households have been observed to be greater than 50% [1].
Spatial and age incidence distributions
58.4% (517) of the AHC cases were males and 41.6% (369) were females. The outbreak focused on coastal cities (93.6% of total cases) as follows: Manzanillo (43.34%) , Tecoman (43.45%), Armeria (6.8%), Colima (3.27%) 4), and Villa de Alvarez (2.48%). The mean age of incidence was 29.28 years (SD 15.88). Figure 2 shows the geographic and age distribution of AHC cases.
Relative frequency of symptoms
The most common reported symptom was excessive tearing of the eye or epiphora (92.9%) followed by conjunctival hyperemia (65.8%), photophobia (54.1%), subconjunctival hemorrhage (48.1%), eye pain (47.5%), and palpebral chemosis (36.4%). Less common symptoms were conjunctival edema (24.2%), blurry vision (10.4%) and fever (9.0%).
Time to diagnosis
The distribution of the times of onset to diagnosis (days) was fitted to an exponential distribution by maximum likelihood estimation methods (Figure 3). The maximum likelihood estimates of the mean and variance for the time of onset to diagnosis were 1.7 days (95% CI 1.59-1.82) and 2.9 days, respectively. The short estimated time from onset to diagnosis is probably due to the easy-to-recognize symptoms characteristic of AHC compared to other diseases like SARS characterized by an initial clinical picture of flu-like symptoms. For instance, the mean time from onset to admission for SARS in Hong Kong was estimated to be approximately 6 days [8], at least three times larger than the mean time of onset to diagnosis of AHC reported here.
| |

Figure 1
A, daily number of new cases by the date of symptom onset during the acute hemorrhagic conjunctivitis (AHC) outbreak in Colima, Mexico (Sep 3-Nov 11) from the clinical records generated by the Mexican Institute of Public Health (IMSS); B, the circles are the cumulative number of AHC cases observed during the initial growth of the outbreak by date of symptom onset. The solid line is the best exponential fit to the data where the intrinsic growth rate r = 0.34.
|
|
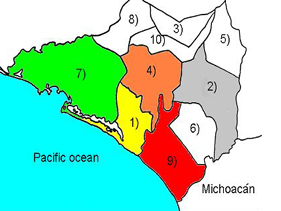
Figure 2
A. Geographic distribution of AHC cases in the state of Colima, Mexico.
1) Armeria (6.8%); 2) Colima (3.27%); 4) Villa de Alvarez (2.48%); 7)
Manzanillo (43.34%); 9) Tecoman (43.45%)
B. Age distribution of AHC cases
(mean 29.28 years; SD 15.88).
|
|
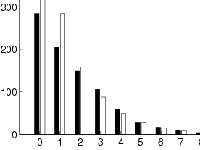
Figure 3.
Observed and estimated maximum likelihood onset to diagnosis intervals. The maximum likelihood estimates of the mean and variance for the time of onset to diagnosis were 1.7 days and 2.9 days, respectively.
|
|
| Discussion | Our estimate for the basic reproductive number for AHC (R0 = 4) is larger than that for Ebola Hemorrhagic Fever [8] (R0=2) a re-emergent infectious disease that is transmitted in a similar fashion. This is probably due to the high mortality rate associated with Ebola outbreaks (50%-90% [10]). While Ebola outbreaks have been observed to last around four months [9], AHC outbreaks last for about two months [1]. This can be attributed to the larger incubation period characteristic of Ebola (up to 21 days [11]) which slows the clinical course of the disease.
Even though AHC is a benign re-emergent infectious disease, its control should be taken seriously as AHC outbreaks can have important economic consequences. The facility to recognize its symptoms (and consequently shorter time to diagnosis, Figure 3) is an advantage from the public health perspective. From our expression for the basic reproductive number, public health measures aiming at reducing the intrinsic growth rate such as increasing hand washing and avoiding direct or indirect contact (object sharing) with infectious cases are promising measures of control. Proper diagnosis of infectious cases guarantees education on how to avoid contagion. All persons with AHC should be cautioned against sharing towels, glasses, goggles, or any other item that could potentially come into contact with the eyes of another individual. Moreover, public health information shopuld be disseminated by press release and distributed as fact sheets in public health clinics, schools, and work places. Infectious cases should remain away from work or school while symptoms persist. School closures have also shown to be effective in reducing contact rates. During the 2002 AHC epidemic in South Korea 1100 schools were closed [4].
It has been observed that full recovery from AHC is achieved within 5-7 days [1]. During the AHC outbreak reported here, the Mexican Institute of Public Health granted three sick days (isolation period at home) to each infected worker (P. Diaz, Mexican Institute of Public Health, Colima, Mex., personal communication). Our estimate for the mean time from onset of symptoms to diagnosis of infectious individuals is 1.7 days (95% CI 1.59-1.82). Hence, we can conclude that the 3 sick-day period granted by the Mexican Institute of Public Health was appropriate for full recovery of infectious individuals while in isolation. It is clear that placing individuals in isolation periods that ensure full recovery can significantly reduce both the final size of an epidemic and its tightly linked economic impact.
| | | Acknowledgements |
This work has been supported through the National Science Foundation grant No. H982300410068 and the National Security Agency grant No. 0441114 to Carlos Castillo-Chavez.
| | | References |
1. CDC. Acute Hemorrhagic Conjunctivitis -- St. Croix, U.S. Virgin Islands, September-October 1998. MMWR 1998; 47:899-901.
2. Hierholzer JC, Hatch MH. Acute hemorrhagic conjunctivitis. In: Darrell RW, ed. Viral diseases of the eye. Philadelphia: Lea \& Febiger, 1985:165-96.
3. R.S. Maitreyi, L. Dar, A. Muthukumar, M. Vajpayee, I. Xess, R.B. Vajpayee, P. Seth, and S. Broor. Acute Hemorrhagic Conjunctivitis Due to Enterovirus 70 in India. Emerg Infect Dis 1997;8:276-9.
4. M.-D. Oh, S. Park, Y. Choi, H. Kim, K. Lee, W. Park, Y. Yoo, E.-C. Kim, and K. Choe. Acute Hemorrhagic Fever caused by cosxackievirus A24 variant, 36 Korea, 2002. Emerg Infect Dis 2003;9:1010-12.
5. Epidemiological Bulletin of Mexico, Number 38, 2003.
6. Milenio.com (Sun 28 Dec 2003). Conjunctivitis - Mexico (Jalisco). ProMed. Dec 28, 2003. Accessed at: http://www.promedmail.org/, archive 20031228.3154.
7. INEGI (Instituto Nacional de Estadistica, Geografia e Informatica). Conteo de poblacion y vivienda, 1995.
8. Donnelly, CA et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. The Lancet 2003;361:1761-1766.
9. Chowell, G., Hengartner, N., Castillo-Chavez, C., Fenimore P.W., et al. The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. Journal of Theoretical Biology 2004; 229: 119-126.
10. World Health Organization (WHO). Ebola Hemorrhagic Fever. http://www.who.int/csr/disease/ebola/en/, accessed on May 21, 2003.
11. Breman, JG, Piot, P, Johnson, KM, et al. The Epidemiology of Ebola Hemorrhagic Fever in Zaire, 1976. Proc Int Colloquium on Ebola Virus Inf held in Antwerp, Belgium, 6-8 December 1977.
| |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in