|
|
 |
 |
 |
 |
|
|
Proton Beam Irradiation for the Treatment of Uveal Melanoma
Digital Journal of Ophthalmology 1997
Volume 3, Number 2
April 30, 1997
|
Printer Friendly
|



Paul Gaudio, B.S.L. | Retina Service, Massachusetts Eye and Ear Infirmary, Harvard Medical School Michael Tolentino, M.D. | Massachusetts Eye and Ear Infirmary, Harvard Medical School Evangelos S. Gragoudas, M.D. | Massachusetts Eye and Ear Infirmary, Harvard Medical School
|
|
|
| Abstract | Objective
To review the history, process and prognosis associated with proton beam irradiation for the treatment of uveal melanoma. | | | Introduction | The utility of ionizing radiation for destroying tumor tissue is well known. Ionizing radiation may be applied in electromagnetic or charged particle form.(1)Charged atomic particles such as protons and helium ions travel in straight lines and exhibit minimal scatter. (2,3)Their well defined stopping point correlates with their initial energy, and they reach maximum ionization density at the end of their path, a point termed the "Bragg Peak."(2-5) Adjustment of the initial energy of a collimated proton beam allows precise targetting of this Bragg peak on the tumor, causing maximum damage where desired, with minimal collateral tissue effect. This form of irradiation is particularly suited to treating tumors which can be well visualized and precisely localized, such as those in the eye.(6)
HISTORY
Considerable early work demonstrated the effectiveness of treating ocular tumors with ionizing radiation applied by various techniques. However, the use of photon(7) and electron(8) radiation for treating retinoblastoma, and cobalt plaques,(9) encapsulated gold,(10) and 106 Ru beta-ray applicators(11) against uveal melanomas led to high complication rates,(4,8,9,12-14) largely because such techniques do not allow for sufficiently precise targeting.
An early therapeutic application of proton beam irradation was to pituitary tumors causing acromegaly(15 )and Cushing's disease.(16 )Experimental work on monkeys demonstrated that the Bragg peak of small-diameter cllimated beams can be positioned on the fundus, producing lesions that are confined to the intended irradiation fields.(2,17 ) Irradiated monkey eyes followed for more than 3.5 years after proton irradiation showed normal retinal architecture as close as 4mm FROM the edge of the sharply demarcated lesions produced by irradiation.(18)
Proton beam irradiation was first used for treating uveal melanomas in July, 1975, at the Harvard Cyclotron in Cambridge, Massachusetts (6), The technique was quickly noted to have favorable results in causing tumor regression while preserving the eye,(3,4,6,19-22 )and by 1987 over a thousand cases had been treated at the Harvard cyclotron alone,(23 ) along with several other cases in Switzerland and the Soviet Union.(24,25) | | | Materials and Methods | THE TREATMENT PROCESS
After some early modifications to improve immobilization of the patient and targeting of the proton beam,(3,4) the current technique has been essentially unchanged since the late 1970s. A patient with a uveal melanoma who elects proton beam irradiation (the other options being radioactive plaque therapy or enucleation(26)) is admitted to the Massachusetts Eye and Ear Infirmary approximately two weeks prior to the scheduled date of irradiation. In the surgical suite, the tumor is localized using transillumination and indirect ophthalmoscopy, and four or five tantalum rings measuring 2.5 millimeters in diameter are sutured to the sclera to serve as references marking the tumor edges. Tumors located far anteriorly are diagrammed using precise measurements, sparing the tantalum rings.(19-21)
During the irradiation sessions, the patient is seated and the head is immobilized using a custom-molded plastic mask (figure 1). Lid retractors are used to remove the lid FROM the radiation field, and the patient is asked to fix his or her gaze on a light attached to the proton collimator. The eye is viewed on a closed circuit television camera using high magnification, to detect any deviation FROM the indicated direction of gaze. A fluoroscopic imaging system is used to verify the eye's precise position during the treatment.(3,19)
A computer planning program is used for targeting the proton beam. Provided with the necessary data, the computer generates a three-dimensional model of the eye and tumor which can be viewed FROM any angle desired (figure 2), while also calculating the best direction of gaze, ideal shape of the field defining apertures, proton range and range modulations, what structures lie in the beam's path, and what should be seen in the alignment film used when aiming the beam.(19)
A total of 7,000 cobalt rad equivalents is given in five sessions over eight to ten days.
All patients undergo systemic screening for metastases by an internist, with chest roentgenograms and liver function studies.(20) Documentation of tumor growth is required for any tumor 10 mm or smaller in diameter or two millimeters or less in height.(20 )Tumors as large as 24mm in diameter have been treated.(19)
Patients are typically seen for follow-up six weeks after treatment, then every four months for the first year, with subsequent visits every six months to every year. The tumor is monitored using fundus photography (figure 3a and b: before and after) and ultrasonography. Liver function studies are recommended annually.(20) | |

Figure 1
A patient about to receive proton beam irradiation.
|
|
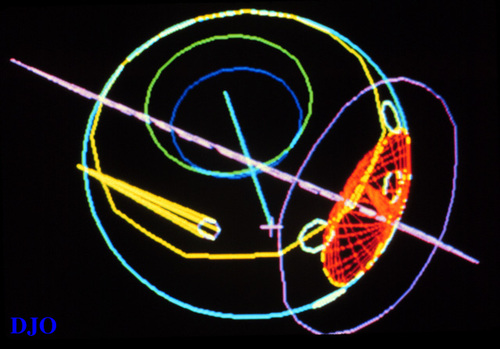
Figure 2
A computer generated three-dimensional model of a the eye with a tumor.
|
|
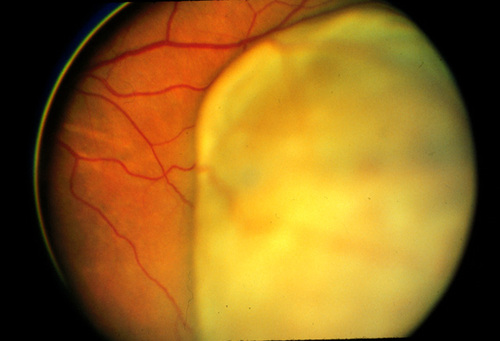
Figure 3a
Uveal melanoma before treatment with proton beam irradiation.
|
|

Figure 3b
Uveal Melanoma after treament with proton beam irradiation
|
|
| Results | | Proton beam irradiation has become established as a treatment for uveal melanoma allowing preservation of the eye with a reasonable prognosis for continued vision, and no increased risk of metastasis. While not without complications, the physical nature of proton beams allows more precise application of ionizing radiation than other methods. | | | Discussion | CLINICAL
The prognosis for vision after proton beam treatment is difficult to predict for the individual patient because of a multitude of confounding factors, including variation in follow-up time between patients, differing baseline vision, size and location of tumor, and presence or absence of the crystalline lens. This question has nevertheless been extensively studied among the Harvard cyclotron treated patients.(22,27-29) A study of 128 patients followed for a median time of 5.4 years post-irradiation found that 58% of them had visual acuity better than 20/100 at last follow-up, while 35% had better than 20/40. Patients who began treatment with better than 20/200 vision were predicted to have a 70% chance of retaining that level after five years, based on a Kaplan-Meier cumulative probability curve.(22)
Tumor location and subsequent irradiation near the optic disc or fovea may logically be expected to adversely affect visual outcome, and several studies have confirmed this.(22,27,28)A study of 562 followed a median of 1.7 years after irradiation determined two-year cumulative rates of visual loss (to vision worse than 20/200) of 47% for eyes with tumors within two disc diameters of the optic disc or fovea, compared with 28% for eyes harboring tumors more anteriorly located. No correlations were found for tumors at varying distances within two disc diameters FROM the disc or macula, and neither structure was better correlated with visual loss than the other.
Tumor height has been correlated inversely with post-irradiation visual acuity, with five millimeters being the point at which significant decline is noted in most studies. A height of eight millimeters is associated with an even worse visual prognosis.(22, 27) Irradiation exposure of the lens is widely known to cause visual impairment secondary to cataract,(22,28,30,31) and eyes in which the lens could not be spared significant proton beam exposure were found to suffer accordingly.(27)
Retinal detachment, optic neuropathy, radiation retinopathy, neovascular glaucoma, vitreous hemorrhage and cataract have emerged consistently as causes of visual loss in proton beam irradiated eyes,(22,27,28 )Occasional difficulties arise in sorting out which of these causes is most responsible in any given case, as they may occur in the same patient in whom the presence of a cataract may further hamper a view of the fundus.(28)
Many patients, even when made desperately aware of a poor prognosis for retaining vision, elect proton beam treatment to avoid losing their eye. At times, however, eventual enucleation has been required despite proton beam therapy. A 1989 study of 994 patients generated a Kaplan-Meier cumulative probability estimate of 10% risk of enucleation by five years post-irradiation.(32) Sixty four (6.4%) treated eyes actually were enucleated within a median of 2.7 years follow-up. The highest risk occurred during the first year, when 3% of enucleations were performed, then declining to 1% per year. By multivariate analysis, it was found that predicting factors for enucleation after proton beam treatment were: a) tumor distance of two or fewer disc diameters FROM the optic disc or fovea; b) involvement of the ciliary body by tumor; c) tumor height greater than eight millimeters. Leading indications for enucleation were neovascular glaucoma (47% of cases), continued tumor growth (25%), retinal detachment (14%), and angle closure glaucoma not due to neovascularization (6%). None of these were associated with a particular duration of time following irradiation in this important study.(32)
A 1986 study determined a 20% cumulative risk of developing metastasis by five years after irradiation, based on 128 patients followed a median of 5.4 years.(22) In a study of 780 eyes (which included many of the 128 noted above) followed a median of 2.2 years, eight percent of that study population actually developed metastases, the median time to detection of metastases being 2.1 years.33 Based on Kaplan-Meier probability curves, the same five-year estimated 20% cumulative risk of metastasis was determined. Eighty to 90 percent of metastases FROM uveal melanoma have occurred in the liver in these patients,(22,33,34 )less common sites being the skin and lung.
Tumor involvement of the ciliary body, tumor diameter of greater than 15 mm, extrascleral extension of the tumor, and patient age greater than 60 years have all been statistically correlated with subsequent metastases in the above cited studies. Pregnancy and the use of birth control pills in women who were diagnosed with uveal melanoma and received proton beam therapy was found not to influence the risk of subsequent metastasis.(35)
The prognosis for patients with metastatic disease FROM uveal melanoma is poor. There was a one year survival of 13% among 145 patients diagnosed with metastasis after proton beam therapy.(36) The median time to diagnosis was 2.4 years post-irradiation, and the median survival was two months among untreated patients. Treatment for metastasis increased the median duration to five months.(36) A meticulous statistical comparison of 556 patients who underwent proton beam irradiation versus two groups of approximately 240 patients who underwent enucleation as treatment for uveal melanoma suggested that treatment choice of either irradiation or metastasis had little overall influence on survival in this disease, despite the acknowledged difficulties inherent in such a comparison.(37,38)
The likelihood of tumor regression clearly merits consideration when evaluating proton beam therapy as a treatment modality. This likelihood has proven small; a five year cumulative estimate of three percent was generated by a study of 1077 patients followed a mean of 48 months.(23) The median time to diagnosis of tumor regrowth was 19 months in 20 tumors (1.9%) that showed growth. Tumor diameter greater than 15 mm, tumor height greater than five millimeters, ciliary body involvement by the tumor, and male sex were all correlated with tumor growth. Five of the nine ciliary body tumors in this study were ring melanomas not diagnosed as such before irradiation, and tumor size may be explained by the fact that larger tumors may have more areas of hypoxia and therefore might be less susceptible to radiation damage, but the significance of the male sex correlation in this study is unclear.
HISTOPATHOLOGICAL
Studies of the histopathologic changes produced by proton beam irradiation have been informative. Vascular changes within the tumor and in adjacent irradiated tissue are among the most commonly seen.(39-44) These include swelling of endothelial cells,(39,40) basement membrane thickening with retinal vascular occlusion,(39-41) and sinusoidal collapse. Damage to the tumor tissue itself is shown to take the form of lipid vacuolization of tumor cells, pyknotic nuclei, mitochondrial breakdown, melanin deposition and balloon cell formation.(39-44) Areas of necrosis with proteinaceous exudate and pigment laden macrophages are also shown to occur, along with fibrous metaplasia of the retinal pigment epithelium. One study involving three eyes noted that the degree to which these changes occurred correlated inversely with time after irradiation.(39)
Mitotic activity in the enucleated eyes studied has been scant; always fewer than 11, and most often fewer than three mitoses per 40 per high power fields have been noted in the published reports.(39,41-44)
COMPLICATIONS
Clinically noted complications have been the subject of considerable attention in studies of proton beam irradiated eyes. Radiation maculopathy has been found to be a common complication after irradiation of paramacular tumors; it was noted in 89% of a series of 218 such patients.(45) This maculopathy, characterized by microvascular changes in 76% of eyes studied, retinal hemorrhages in 70%, and capillary non-perfusion in 64%, resolved in only five percent of cases.
Forty two percent of 383 patients who received proton beam irradiation for uveal melanoma developed new posterior subcapsular opacities, in direct proportion to the dose of radiation received by the lens in the irradiated eye--as determined by a computer-generated dose-volume assessment routinely calculated at the time of irradiation.(30 )Cortical and sclerotic opacities were found correlated only to age in this study.
Cataract extraction in eyes which have undergone proton beam therapy has been successful and not associated with increased risk of metastasis. A study of such eyes with a mean time of 3.1 years between irradiation and extraction, found some visual improvement after extraction.(47)
FUTURE INVESTIGATION
Proton beam therapy may eventually be complemented by other evolving techniques. A 1985 study demonstrated the efficacy of applying subtherapeutic doses of proton beam irradiation and ultrasonically induced hyperthermia to treat experimentally generated choroidal melanomas in rabbits.(48) The possibility of studying whether adjuvant chemotherapy can reduce tumor related mortality in patients with uveal melanomas is presently under investigation. It is doubtless that continued refinement of current methods and development of new techniques will further improve the outlook of patients with uveal melanomas. | | | References | 1. Wyngaarden JB, Smith LH [eds.]. Cecil Textbook of Medicine, 18th ed., Vol I. Philadelphia, WB Saunders Co., 1988, p. 1115.
2. Constable IJ, Koehler AM. Experimental ocular irradiation with accelerated protons. INVEST OPHTHALMOL 1974;113:280-7.
3. Gragoudas ES, Goitein M, Verhey L, Munzenrider JE, Suit H, Koehler A. Proton beam irradiation, an alternative to enucleation for intraocular melanomas. OPHTHALMOL 1980;87:571-80.
4. Gragoudas ES, Goitein M, Koehler AM, et al. Proton irradiation of choroidal melanomas, preliminary results. ARCH OPHTHALMOL 1978;96:1583-91.
5. Wilson RR. Radiological use of fast protons. RADIOL 1946;47:547.
6. Gragoudas ES, Goitein M, Koehler AM, et al. Proton irradiation of small choroidal malignant melanomas. AM J OPHTHALMOL 1977;83:665-73.
7. Reese AB, Merriam GR, Martin HE. Treatment of bilateral retinoblastoma by irradiation and surgery; report on 15 year results. AM J OPHTHALMOL 1949;32:175-80.
8. Hultberg S, Walstam R, Asard PE. Two special applications of high-energy electron beams. ACTA RADIOL 1965;3:287-90.
9. Long RS, Balin MA, Rotman M. Conservative treatment of intra-ocular melanomas. TRANS AM ACAD OPHTHALMOL OTOLARYNGOL 1971.75:84-93.
10. Newman GH, Davidorf FH, Havener WH, et al. Conservative management of malignant melanoma: I. Irradiation as a method of treatment for malignant melanoma of the choroid. ARCH OPHTHALMOL 1970;83:21-26.
11. Lommatzsch PK. Experiences in the treatment of malignant melanoma of the choroid with 106Ru-106Ru beta ray applicators. TRANS OPHTHALMOL SOC UK 1973;93:119-32.
12. Reese AB, Ellsworth RM. Management of retinoblastoma. ANN NY ACAD SCI 1964;114:958-63.
13. Bedford MA, Bedotto C, Macfaul PA. Radiation retinopathy after the application of a cobalt plaque: report of three cases. BR J OPHTHALMOL 1970;54:505-9.
14. Macfaul PA, Bedford MA. Ocular complications after therapeutic irradiation. BR J OPHTHALMOL 1970;54:237-47.
15. Kjellberg RN, Shintani A, Frantz AG, et al. Proton-beam therapy in acromegaly. N ENGL J MED 1969;287:689-96.
16. Falkner S, Fors B, Larsson B. Pilot study on proton irradiation of human carcinoma. ACTA RADIOL 1962;58:33-40.
17. Constable IJ, Koehler AM, Schmidt RA. Proton irradiation of simulated ocular tumors. INVEST OPHTHALMOL 1975;14:547-55.
18. Gragoudas ES, Zakov NZ, Albert DM, Constable IJ. Long-term observations of proton-irradiated monkey eyes. ARCH OPHTHALMOL 1979;97: 2184-91.
19. Gragoudas ES, Goitein M, Verhey L, et al. Proton beam irradiation of uveal melanomas, results of a 5 1/2 year study. ARCH OPHTHALMOL 1982;100:928-34.
20. Gragoudas ES, Seddon J, Goitein M, et al. Current results of proton beam irradiation of uveal melanomas. OPHTHALMOL 1985;92:284-91.
21. Gragoudas ES, Goitein M, Koehler A, et al. Proton irradiation of malignant melanoma of the ciliary body. BR J OPHTHALMOL 1979;63:135-9.
22. Gragoudas ES, Seddon JM, Egan KM, et al. Long-term results of proton beam irradiated uveal melanomas. OPHTHALMOL 1987;94:349-53.
23. Gragoudas ES, Egan KM, Seddon JM, Walsh SM. Munzenrider JE. Intraocular recurrence of uveal melanoma after proton beam irradiation. OPHTHALMOL 1992;99:760-6.
24. Zografos L, Gailloud CL, Perret CH, et al. Rapport sur le traitement conservateur des melanomes de l'uvee a la clinique ophthalmologique universitaire de Lausanne. KLIN MONATSBL AUGENHEILKD 1988;192:572-8.
25. Brovkina AF, Zarubei GD. Ciliochoroidal melanomas treated with a narrow medical proton beam. ARCH OPHTHALMOL 1986;104:402-4.
26. Gragoudas ES. Current approaches in the management of uveal melanomas. INT OPHTHALMOL CLIN 1992;32:129-38.
27. Seddon JM, Gragoudas ES, Polivogianis L, et al. Visual outcome after proton beam irradiation of uveal melanoma. OPHTHALMOL 1986;93:666-74.
28. Seddon JM, Gragoudas ES, Egan KM, et al. Uveal melanomas near the optic disc or fovea, visual results after proton beam irradiation. OPHTHALMOL 1987;94:354-61.
29. Park SS, Walsh SM, Gragoudas ES. Visual-field deficits associated with proton beam irradiation for parapillary choroidal melanoma. OPHTHALMOL 1996;103:110-6.
30. Gragoudas ES, Egan K, Walsh SM, Regan S, Munzenrider JE, Taratuta V. Lens changes after proton beam irradiation for uveal melanoma. AM J OPHTHALMOL 1995;119:157-64.
31. Cogan DC, Donaldson DD, Reese AB. Clinical and pathological characteristics of radiation cataract. ARCH OPHTHALMOL 1952;47:55-70.
32. Egan KM, Gragoudas ES, Seddon JM, et al. The risk of enucleation after proton beam irradiation of uveal melanoma. OPHTHALMOL 1989;96:1377-83.
33. Gragoudas ES, Seddon JM, Egan KM, et al. Metastasis FROM uveal melanoma after proton beam irradiation. OPHTHALMOL 1988;95:992-9.
34. Gragoudas ES, Seddon JM, Egan KM, et al. Prognostic factors for metastasis following proton beam irradiation of uveal melanomas. OPHTHALMOL 1986;93:665-80.
35. Egan KM, Walsh SM, Seddon JM, Gragoudas ES. An evaluation of the influence of reproductive factors on the risk of metastasis FROM uveal melanoma. OPHTHALMOL 1993;100:1160-5.
36. Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastasis FROM uveal melanoma. OPHTHALMOL 1991;98:383-90.
37. Seddon JM, Gragoudas ES, Egan KM, et al. Relative survival rates after alternative therapies for uveal melanoma. OPHTHALMOL 1990;97:769-77.
38. MacLean IW. Discussion of: Relative survival rates after alternative therapies for uveal melanoma. OPHTHALMOL 1990;97:777.
39. Seddon JM, Gragoudas ES, Albert DM. Ciliary body and choroidal melanomas treated by proton beam irradiation, histopathologic study of eyes. ARCH OPHTHALMOL 1983;101:1402-8.
40. Zinn KM, Stein/Pokorny K, Jakobiec FA, et al. Proton beam irradiated epithelioid cell melanoma of the ciliary body. OPHTHALMOL 1981;88:1315-21.
41. Ferry AP, Blair DJ, Gragoudas ES, Volk SC. Pathologic examination of ciliary body melanoma treated with proton beam irradiation. ARCH OPHTHALMOL 1985;103:1849-53.
42. Kincaid MC, Folberg R, Torczynski E, et al. Complications after proton beam therapy for uveal malignant melanoma. OPHTHALMOL 1988;95:982-91.
43. Saornil MA, Egan KM, Gragoudas ES, Seddon JM, Walsh SM, Albert DM. Histopathology of proton beam-irradiated vs. enucleated uveal melanomas. ARCH OPHTHALMOL 1992;110:1112-8.
44. Young LHY, Gragoudas ES. Macular uveal melanoma treated with proton beam irradiation, 10 year follow-up observation with histopathologic correlation. RETINA 1994;14:43-6.
45. Margo CE, Pautler SE. Granulomatous uveitis after treatment of a choroidal melanoma with proton beam irradiation. RETINA 1990;10:150-3.
46. Guyer DR. Mukai S, Egan KM, Seddon JM, Walsh SM, Gragoudas ES. Radiation maculopathy after proton beam irradiation for choroidal melanoma. OPHTHALMOL 1992;99:1278-85.
47. Gragoudas ES, Egan KM, Arrigg, PG, Seddon JM, Glynn RJ, Munzenrider JE. Cataract extraction after proton beam irradiation for malignant melanoma of the eye. ARCH OPHTHALMOL 1992;110:475-9.
48. Riedel KG, Svitra PP, Seddon JS, et al. Proton beam irradiation and hyperthermia, effects on experimental choroidal melanoma. ARCH OPHTHALMOL 1985;103:1862-9. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in