|
|
 |
 |
 |
 |
|
|
A Review of Diabetic Macular Edema
Digital Journal of Ophthalmology 1997
Volume 3, Number 6
July 1, 1997
|
Printer Friendly
|
|
|
|
|
| Abstract | Objective
This article will provide a review of the essential aspects of diabetic macular edema. The established criteria for classifying and diagnosing this condition, as well as specific treatment strategies will be addressed. However, it is necessary to first understand the magnitude of the problem that diabetic macular edema represents, and the underlying pathophysiology of the clinical findings. | | | Introduction | EPIDEMIOLOGY: DEFINING THE PROBLEM
Up to 10 % of all patients with diabetes will develop diabetic macular edema (DME) during their lifetime. The magnitude of this problem can be appreciated by considering that, since diabetes is a relatively common systemic disease, this means that approximately 500,000 Americans have macular edema. Up to 75,000 new cases of diabetic macular edema develop each year. Many cases have a significant effect on visual acuity: up to 4% of diabetic patients develop DME that affects the central fovea; up to 30 % of patients with clinically significant macular edema (which will be defined later on) will develop moderate visual loss (a doubling of the visual angle).
Relationship to Ocular and Systemic Disease
It is important to realize that diabetic macular edema is not a condition that develops independently FROM other diabetic findings. Rather, it is closely associated with the degree of diabetic retinopathy that is present, and the duration and type of diabetes that the patient has. In patients with mild non-proliferative diabetic retinopathy (NPDR), there is a 3% incidence of DME. Those with moderate to severe NPDR have a 40% incidence of DME. In the presence of proliferative diabetic retinopathy (PDR) there is a 71% incidence of DME.
Regarding the duration and type of diabetes: patients with insulin dependent diabetes mellitus (IDDM) of ten years duration have a 7-10% incidence of diabetic macular edema. Someone who has had IDDM for 20 years has a 25-30% incidence of DME. Macular edema is rare in a patient who has had IDDM for less than eight years. The association between DME and non-insulin dependent diabetes mellitus (NIDDM) depends not only on the duration of the disease, but also on the use of insulin. For patients with NIDDM who are also on insulin, a ten year disease duration is associated with a 10 % incidence of DME, while a 20 year disease duration has a 30-35% incidence of DME. Those with NIDDM who do not take insulin have a 5 % incidence of DME if they have had the disease for 10 years, and a 15% incidence of DME if they have had NIDDM for 20 years.
PATHOPHYSIOLOGY: UNDERSTANDING THE PROBLEM
The defining feature of diabetic macular edema is, of course, edema of the retina. It is seen clinically as thickening of the layers of the retina, and arises as a result of an abnormal accumulation of fluid within the retina. Edema arises due to a disruption of the balance between capillary/vascular hydrostatic forces and plasma/tissue oncotic pressure gradients. The relationship is similar to those found in other vascular systems in the body. Increasing intravascular or intracapillary hydrostatic forces favors the net movement of fluid INTO the surrounding tissues. The oncotic pressure gradients, largely dependent on plasma protein levels, also determine the net movement of intravascular, intercellular, and intracellular fluid. However, a unique feature of the eye that enters the equation is the blood-retinal barrier (BRB). The retinal capillaries constitute the inner BRB, and the retinal pigment epithelium (RPE) comprises the outer BRB. Retinal capillary abnormalities disrupt the inner BRB, and can affect any hydrostatic or oncotic gradients that would normally exist. RPE disease may similarly affect the outer BRB.
Systemic Conditions
In addition to local ocular conditions, systemic diseases, especially those that are commonly found in association with diabetes, can also have an affect on the development and severity of diabetic macular edema. Severe hypertension or fluid retention FROM any cause increases capillary hydrostatic pressure which in turns drives fluid FROM the vascular spaces INTO the surrounding retina. Hypoalbuminemia is frequently found in diabetic patients as a result significant renal disease and subsequent loss of protein in the urine (proteinuria). The decreased plasma protein concentration decreases the intravascular oncotic pressure. This in turn results in net fluid movement INTO the retinal tissues. These systemic diseases can worsen DME, and their treatment and control can help DME resolve [see fig.1].
Focal and Diffuse Diabetic Macular Edema
Diabetic macular edema can be classified INTO focal and diffuse types. This is a very useful classification since the two types vary significantly in their clinical appearance, method of treatment, and underlying pathophysiology. Focal macular edema is caused by foci of vascular abnormalities, primarily microaneurysms, which have an abnormally high permeability [see fig. 2a]. Diffuse macular edema is caused by dilated retinal capillaries throughout the posterior pole. Widespread retinal non-perfusion results in compensatory dilatation of the remaining capillary bed. In diffuse macular edema, cystoid retinal edema is more common. It is also felt that some leakage of fluid may occur across the RPE [see fig.2b].
Hard Exudates
Hard exudates are one of the most common findings in DME. However, it is essential to understand that the presence of hard exudates is not required to diagnose DME. That being said, hard exudates can provide important clues as to the existence and evolution of DME, particularly if one does not have stereo photographs available [see fig.3]. Hard exudates represent lipoprotein accumulations in the inner and outer plexiform layers of the retina. Sub-retinal accumulation of hard exudates can also occur and cause photoreceptor damage and/or RPE metaplasia resulting in sub-retinal fibrosis. Hard exudates are less common in diffuse DME -- diffuse BRB breakdown may not allow the passage of the large lipoprotein molecules INTO the retinal layers.
| | | Results | | The following points summarize the approach to the patient with diabetic macular edema. The underlying pathophysiology helps us to understand how it develops and how we can treat it. The diagnosis is made on clinical grounds only; the fluorescein angiogram is used only to guide the treatment. The ETDRS provides valuable guidance on how, when, and why to treat DME. It is also important to remember that the patient's systemic condition can have a significant effect on DME. By considering these points, we can often be of great assistance to patients with this common, but treatable, ophthalmologic condition. | | | Discussion | EARLY TREATMENT DIABETIC RETINOPATHY STUDY: SOLVING THE PROBLEM
The Early Treatment Diabetic Retinopathy Study (ETDRS) set the standard of care for managing diabetic macular edema. One of the most significant medical trials ever conducted, it continues to provide new and useful clinical information: the latest report FROM the trial was published in September 1996. The ETDRS was a multi-center collaborative clinical trial supported by National Eye Institute. It involved 3928 patients enrolled FROM 1979-1989.
Goals and Design of the Study
The ETDRS had three primary goals: to determine when it is most effective to initiate scatter or pan-retinal photocoagulation (PRP); to investigate the effect of aspirin on diabetic retinopathy; and to determine if laser photocoagulation is an effective treatment for diabetic macular edema. Only those aspects of the study pertaining to DME will be discussed in detail.
Patients enrolled in the study were required to have a visual acuity of at least 20/40, or 20/400 if the decreased acuity was due to DME. All eyes had to have mild NPDR to non high-risk PDR (as defined by the earlier Diabetic Retinopathy Study) with or without DME. One eye of each patient was randomized to deferral of laser treatment unless high-risk PDR develops (since the Diabetic Retinopathy Study had already demonstrated the efficacy of treatment under these conditions). The other eye was assigned to early photocoagulation. A total of 1489 eyes with mild to moderate NPDR and DME were assigned to deferred treatment. Another 754 similar eyes received immediate treatment for DME only (as well as scatter retinal photocoagulation treatment only if severe NPDR or PDR developed later). It is these two groups ( that is, the 1489 eyes versus the 754 eyes) that were compared in the study to determine the efficacy of laser treatment for DME.
PATHOPHYSIOLOGY: UNDERSTANDING THE PROBLEM
Clinically Significant Macular Edema
The definition of clinically significant macular edema (CSME) is the most significant outcome of the ETDRS in that it established a method for classifying and diagnosing DME and determining when treatment is required. In ORDER to diagnose CSME, one of the following characteristics must be present on clinical examination:
Any retinal thickening within 500 microns of the center of the macula.
Hard exudates within 500 microns of the center of the macula with adjacent retinal thickening.
Retinal thickening at least 1 disc area in size, any part of which is within 1 disc diameter of the center of the macula.
It is essential to realize that the determination of the presence of CSME is a clinical diagnosis based on a retinal biomicroscopic examination of the patient, and not based on fluorescein angiography. The ETDRS defined moderate visual loss (MVL) as a doubling of the visual angle on two successive follow-up visits. Photocoagulation reduced the risk of MVL in patients with CSME by 50% (30% vs 15% at three years). These patients were also more likely to improve visual acuity by one or more lines.
PHOTOCOAGULATION AND FLUORESCEIN ANGIOGRAPHY: TREATING THE PROBLEM
The ETDRS demonstrated the efficacy of laser photocoagulation in treating CSME. With few exceptions, patients who are diagnosed with CSME should be offered laser treatment. After discussing the risks, alternatives, and benefits of treatment with the patient, a fluorescein angiogram should be performed if the patient agrees to undergo photocoagulation.
Fluorescein angiography
The only indication for fluorescein angiography in relation to DME is to demonstrate lesions and areas of the retina that require treatment once the decision to treat has been made on clinical grounds. The angiogram is used to identify which microaneurysms are leaking and causing the edema and to help locate focal lesions that may not have been obvious on clinical examination [see fig.4a-c]. The angiogram can also determine the extent of retinal non-perfusion which may require treatment [see fig. 5a,b]. It cannot be overstated that fluorescein angiography is not used to diagnose CSME nor to determine if treatment has been successful or needs to be repeated.
Pathophysiology of Laser Treatment
There are two types of laser treatment for DME: focal and grid. Focal laser treatment is, of course, used to treat focal DME. The underlying process is the closure of leaking microaneurysms by thermal coagulation. The laser light must be absorbed by the blood inside the microaneurysms. Green and yellow wavelengths work well for this. However, blue wavelengths are excessively absorbed by the macular xanthophyll and should not be used. Red wavelengths are not well absorbed by blood and will therefore not close microaneurysms well. However, red wavelengths can be useful in treating diffuse DME as will be discussed below.
Grid laser treatment is used to treat diffuse DME. There are several theories to EXPLAIN how grid treatment works. One hypothesis is that the laser energy removes unhealthy RPE cells which are then replaced by more viable RPE cells. It is also possible that grid laser treatment destroys retinal photoreceptors and decreases oxygen consumption, which in turn decreases blood flow within the leaking vessels. Another theory states that photocoagulation stimulates the existing RPE cells to absorb more fluid. Finally, laser treatment may stimulate vascular endothelial proliferation and improve the integrity of the inner blood-retinal barrier. It is likely that a combination of these processes underlies the efficacy of grid laser treatment.
Pathophysiology of DME Resolution
It usually requires 3- 6 months for thickening and hard exudates to resolve. The fluid that is causing the edema is first reabsorbed across the inner and/or outer blood-retinal barrier. The lipoproteins that are responsible for the hard exudates are reabsorbed by macrophages over a longer period of time. When the edema resolves first, the lipids can precipitate out of the remaining fluid and the amount of hard exudates can increase temporarily. It is important to recognize this not uncommon occurrence so that one does not mistakenly believe that the DME is worsening, rather than improving, after treatment [see fig.6a,b].
Laser Treatment
The ETDRS also defined which lesions associated with DME should be treated:
Focal leaks more than 500 microns FROM the center of the macula if causing thickening or hard exudates (HE).
Focal leaks 300 - 500 microns FROM the center of the macula causing thickening or HE if visual acuity is less than 20/40 and the remaining perifoveal capillary net will not be destroyed by the treatment.
Areas of diffuse leakage or avascular zones not previously treated.
Specific treatment strategies were shown in the ETDRS to be most effective. For treating focal leaks, a laser spot size of 50 -100 microns (on the retina) is used. A 50 micron spot size should be used when treating within 500 microns of the center of the macula. A 0.1 second exposure time is typically used. When treating microaneurysms at least 40 microns in size, an actual darkening or whitening of the lesion should be observed which indicates closure of the microaneurysm. Several 50 micron hits with the laser can used if necessary. One should reconfirm closure of these lesions at the end of the treatment session, and retreat the lesions if necessary. It is often difficult to observe actual closure of lesions less than 40 microns in size. For these lesions the goal of treatment should be a mild to moderate whitening of the surrounding retina. Clumps of microaneurysms can be treated using a larger spot size (200-500 microns) followed by 50 micron treatment of individual lesions. It is important to avoid confluent laser treatment in the papillomacular bundle. Nerve fibre layer hemorrhages should be not be treated in ORDER to avoid excessive heating and damage of the nerve fibre layer.
Diffuse DME is treated by a grid pattern of laser. The grid is applied to areas of retinal thickening with diffuse leakage or capillary non-perfusion. A 50-200 micron spot size is utilized. The goal of treatment is to produce a retinal burn of mild to moderate intensity. Laser burns should be placed at least one burn width apart -- wider if thickening is less severe. If necessary, the grid can extend up to 2 disc diameters superiorly, inferiorly, and temporally FROM the center of the macula. One should avoid treating within 500 microns of the disc margin or the center of the macula.
The patient should be rechecked three months after treatment. If the DME is not responding to treatment and CSME is still present, the eye can be re-treated according to the above parameters. Remember that the decision to re-treat is based solely on the clinical examination and not on a fluorescein angiogram. A fluorescein angiogram usually needs to be ordered to guide treatment once the decision to treat has been made. It is also important to remember to consider any systemic conditions that may be affecting the DME. The patient should understand that the goal of treatment is to maintain the current visual acuity and reduce the chances of progressive visual loss. It is important to EXPLAIN to the patient that even after successful treatment the visual acuity often does not improve. | | | Acknowledgements | | The author wishes to thank Bob Cavicchi and Jim Strong for providing the clinical photographs. Thanks also to Lloyd M. Aiello, MD, Lloyd P. Aiello, MD, PhD, Timothy Murtha, MD, and George Sharuk, MD, for providing the clinical cases used to demonstrate the various clinical features discussed in this article. | | | References | 1) Aiello LM: Diagnosis, management, and treatment of nonproliferative diabetic retinopathy and macular edema. In Albert DM, Jakobiec FA, ed. Principles and Practice of Ophthalmology, vol. 2, Retina and Vitreous. Philadelphia, WB Saunders Co., 1994, pp 747-760.
2) Bresnick GH: Background diabetic retinopathy. In Ryan SJ ed. Retina, vol. 2, Medical Retina. St. Louis, CV Mosby, 1989, pp 327-366.
3) Early Treatment Diabetic Retinopathy Study Report Number 1: Photocoagulation for diabetic macular edema. Arch Ophthalmol 103:1796-1806, 1985.
4) Early Treatment Diabetic Retinopathy Study Report Number 2: Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Ophthalmology 94:761-774, 1987.
5) Early Treatment Diabetic Retinopathy Study Report Number 4: Photocoagulation for diabetic macular edema. Int Ophthalmol Clin 27:265-272, 1987.
6) Early Treatment Diabetic Retinopathy Study Report Number 5: Detection of diabetic macular edema. Ophthalmoscopy versus photography. Ophthalmology 96:746-751, 1989.
7) Early Treatment Diabetic Retinopathy Study Report Number 7: Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. Ophthalmology 98:741-756, 1991.
8) Early Treatment Diabetic Retinopathy Study Report Number 22: Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Arch Ophthalmol 114:1079-1084, 1996.
9) Klein R, Klein BEK, Moss SE, et al: The Wisconsin Epidemiologic Study of Diabetic Retinopathy, IV. Diabetic macular edema. Ophthalmology 91:1464-1474, 1984. | |
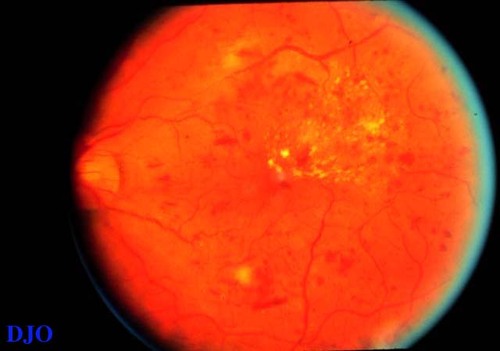
Figure 1
In addition to the widespread macular edema and hard exudates (HE), several nerve fibre layer hemorrhages and cotton-wool spots are present. This patient had severe renal disease with proteinuria and hypertension.
|
|
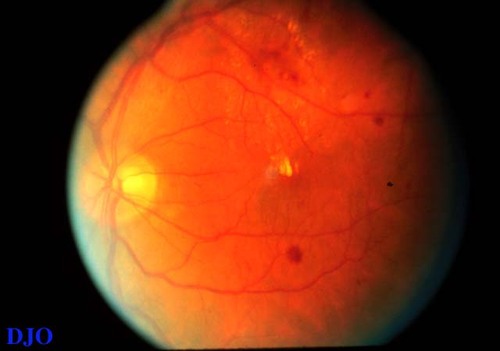
Figure 2a
The findings are typical of focal DME. The hard exudates and edema (difficult to appreciate without stereo photographs) are localized to the area inferior to the fovea. The source of the leakage is likely localized to the area of hemorrhages within the ring of HE near the bottom of the photograph.
|
|
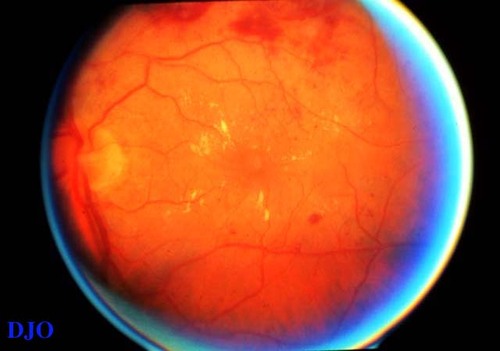
Figure 2b
This shows diffuse DME. The HE and edema are widely distributed around the fovea. Few microaneurysms are seen, which implies that the leakage is due to widespread non-perfusion rather than focal leaks. Cystoid foveal changes are also present, but difficult to detect without a stereo view.
|
|
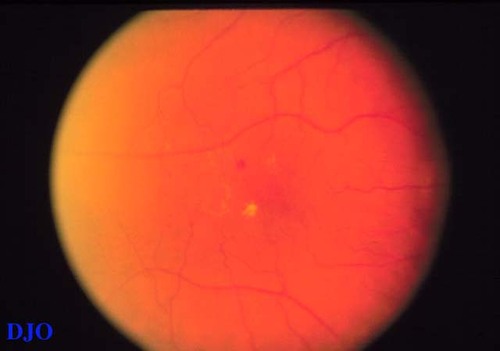
Figure 3
Despite the two-dimensional view of the photograph. The presence and distribution of HE indicates localized edema around the fovea.
|
|
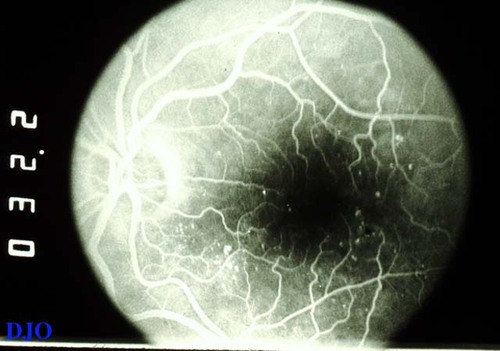
Figure 4a
This is the early frame of a fluorescein angiogram negative ( the fluorescein dye is white). The pinpoint areas of hyperfluorescence correspond to microaneurysms. They are most noticeable in the areas supero-temporal and infero-nasal to the fovea.
|
|

Figure 4b
This later frame shows the microaneurysms beginning to leak. The hyperfluorescence is more intense and diffuse, especially in the two areas previously noted.
|
|
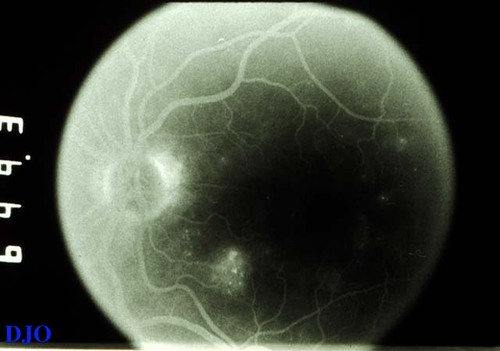
Figure 4c
The late frames of the angiogram shows persistent hyperfluorescence in the area infero-nasal to the fovea. This area of late leakage corresponds to the area of edema that was seen clinically. Mild leakage is noted supero-temporal to the fovea.
|
|
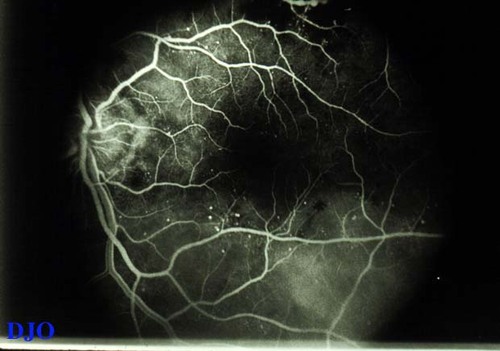
Figure 5a
This early angiogram frame shows enlargement and irregularity of the foveal avascular zone. This represents non-perfusion. Only a few microaneurysms are seen..
|
|
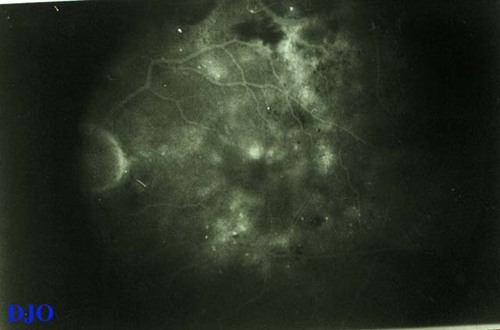
Figure 5b
This late frame shows marked, diffuse hyperfluorescence, but still no signs of significant microaneurysms. This corresponds clinically to diffuse DME. Some cystoid pooling of fluorescein around the fovea, a common finding in diffuse DME, is also seen.
|
|
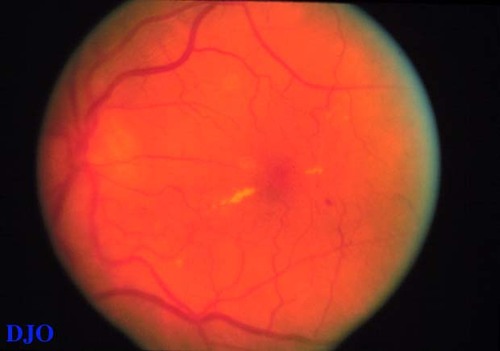
Figure 6a
This patient has focal DME. The area of edema is infero-nasal to the line of hard exudates nasal to the fovea. The area of edema was treated with laser photocoagulation.
|
|
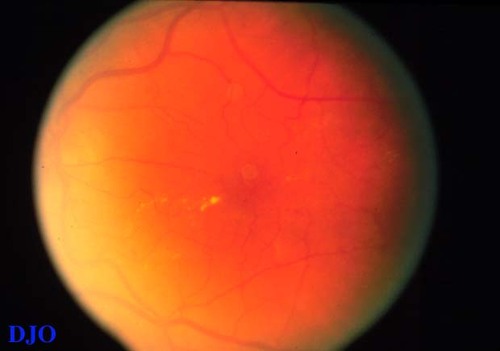
Figure 6b
This photograph was taken 3 months after laser treatment. The area of edema has resolved, but a ring of hard exudates has now developed in the area infero-nasal to the fovea. These exudates represent resolution, not worsening, of the DME.
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in