|
|
 |
 |
 |
 |
|
|
Photoreceptor Degeneration in a Selected Animal Model: The Abyssinian Cat
Digital Journal of Ophthalmology 1998
Volume 4, Number 2
August 15, 1998
|
Printer Friendly
|
|
|
|
|
| Abstract | Keywords
retina, degeneration, photoreceptor, cat, hereditary | | | Introduction | The Abyssinian cat has been shown to be affected by a hereditary retinal degeneration that shares many similarities with human retinitis pigmentosa (RP). The disease was characterized by clinical and laboratory investigations in groups of normal cats and Abyssinian cats affected by ophthalmoscopically obvious disease [1, 2]. In short, the fundus appeared normal until the animals reached young adulthood, at the age of 1.5-2 years, at which time slowly progressive changes began (Figure 1) that led to generalized retinal atrophy by the time the cats were middle-aged [3].
DC-electroretinography (ERG) showed that there was a primary effect by the disease on the function of rod photoreceptors with a later involvement also of the cone system. The function of the retinal pigment epithelium (RPE) was not affected, however, until at a late stage of the degenerative disease process. [4]. These changes were verified by ultrastructural studies which showed changes primarily in solitary rod outer segments or in several rods in patches, while cones and other retinal cells were normal appearing [5]. The morphological changes were more severe in midperipheral and peripheral areas of the retina compared to the central parts, which seemed to be spared until late in the disease, when there was a generalized drop-out of both rods and cone photoreceptors. Through retrospective studies of breeding pairs and their off-spring a simple recessive mode of inheritance for the defect was found [6].
During the last decade the Abyssinian cat model has been further studied in ORDER to more thoroughly characterize the disease as to early functional and morphological changes, including the use of immunohistochemical and immunochemical methods. Biochemical studies of blood and affected tissue have also been performed as well as, more recently, specific physiologic and molecular genetic studies. Furthermore, treatment trials were initiated, utilizing the technique of neuro-retinal transplantation. The present paper aims at summarizing the preliminary findings of these investigations. | |
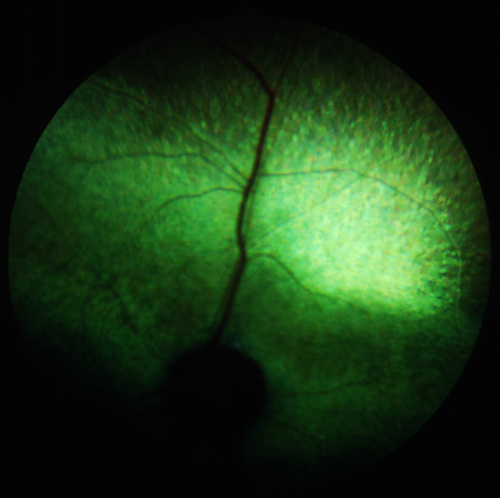
Figure 1
The fundus of an advanced case of hereditary retinal degeneration in a 4-year old Abyssinian cat.
|
|
| Results | The Abyssinian cat mutant has become a well characterized animal model for human RP, which offers the advantage of a large eye that has several similarities to human. Routine ophthalmic examinations can be performed in cat as well as the utilization of medical and surgical techniques regularly in use for human patients. Furthermore, the cat has since long been an established species in the field of basic and applied Neuro-Science, which so far has rendered a great amount of basic scientific knowledge especially in the fields of Neurology and Ophthalmology. It is therefore of great importance that research INTO the Abyssinian cat heredo-degeneration can be further persued. Investigations of priority include:
-To elucidate the gene defect in rdAc
-To persue the issue of retinal transplantation with focus on structural and functional aspects
-To investigate the cone mechanisms in hereditary rod-cone degeneration
-To study the physiology of ocular circulation in retinal heredo-degeneration. | | | Discussion | Developmental studies: Electrophysiology and morphology
Using electroretinography (ERG) 10 young Abyssinian cats, homozygous for the disease but with ophthalmoscopically normal fundi were studied and compared to 11 normal cats, all between the ages of 8-104 weeks [7]. A significant reduction of maximum dark adapted b-wave amplitudes was found in cats already at the age of 8-16 weeks when compared to age-mached controls. At this time there was no major difference between affected and controls in scotopic b-wave threshold or implicit time. Ultrastructural studies [8] in 13 affected and 10 control cats between the ages 4-540 days showed that in affected animals there was an early aberration in the orientation of disc membranes and a disorganization of lamellae in certain rod outer segments. At the age of 35 days a difference between affected kittens and normals was observed. Degenerative changes were seen after the age of 5 months, a time when the feline retina is considered mature [9]. Thus, it was clarified that there were ultrastructural signs of disease in cats homozygous for the defect, already before the time of retinal maturation. However, specific degenerative changes were not observed until after the age of 5 months in affected kittens. The ultrastructural findings correlated with ERG results in that there were early changes in rod photoreceptors and specific drop-out of rods in affected animals already at the time of maturation of the cat retina. The reduced scotopic b-wave amplitude but normal timing characteristics as well as normal sensitivity indicated a drop-out of specific rods in early disease.
Immunocytochemistry
Retinas of 5 young and middle-aged Abyssinian cats, homozygous for the defect, were then studied using antibodies directed against glutamate decarboxylate (GAD), gamma-amino butyric acid (GABA) [10], glial fibrillary acidic protein (GFAP) [11], opsin, transducin alpha, S-antigen, interphotoreceptor retinoid-binding protein (IRBP) and cone outer segments [12]. For GABA and GFAP, changes in immunoreactions were observed in affected cats, that were considered compatible with, and appeared to be secondary to the stage of the retinal degenerative disease. For IRBP, however, the immunoreactivity was found to be much reduced already in the early stage of the photoreceptor degenerative disease. At later stages of disease there was a complete lack of immunoreaction to IRBP. For the other antigens studied no unexpected results were found. It was concluded that the early reduction of IRBP could be one of the factors leading to photoreceptor cell death. IRBP, which normally is found in the interphotoreceptor matrix in large amounts, may act as a buffer system for free retinoids and as a possible transport system, thereby protecting delicate membranes FROM toxic retinoid effects. Membranolytic effects of retinoids are well known and have been reported [13]. Further studies of the levels of IRBP protein and message in affected retinae were performed, well before onset of clinical retinal degeneration, using immunochemical quantitation of IRBP protein and by Northern blotting and slot-blotting of total RNA using a human cDNA probe. Levels of both IRBP protein and message were significantly reduced below normal as early as at 4 weeks of age in affected retinas [14].
Fundusreflectometry
The regional retinal variations and the kinetics of rhodopsin regeneration were measured in affected cats at different stages of the disease using imaging fundus reflectometry (IFR) [15]. The aim of the study was to study the relationship of rhodopsin levels and rod-mediated function, assessed with full-field ERG. Contour maps of regional density differences at different wavelengths of light after bleaching and after rhodopsin regeneration were produced. The absorbance change at different locations was thus obtained. In affected Abyssinian cats, the relationship between rhodopsin and rod ERG threshold was determined by the probability of quantal absorbtion. A pattern of dysfunction of the photoreceptors similar to that found in patients with "regionalized" form of RP was found, where the relationship is explained by decreased quantal absorbtion by the rods.
Biochemistry of fatty acids
Researchers have long sought systemic markers of inherited retinal degenerations in humans and animals. Converse and her colleagues [16] reported abnormalities in plasma lipids and fatty acids among several Scottish families with RP. Results of several studies suggest a systemic abnormality in polyunsaturated fatty acid metabolism in humans with RP. This prompted the examination of the situation also in Abyssinian cats affected with hereditary retinal degeneration. Twenty-eight affected cats were studied and compaired with 13 control cats [17]. The levels of plasma phospholipids were the same for affected and controls. Differences were seen, however, between control and affected groups of cats in the levels of omega 3 polyunsaturated fatty acids. Control animals had significantly higher levels of 20:5 omega 3 and 22:6 omega 3 than affected animals. The level of 22:5 omega 3 was higher in affected animals than in controls; thus, the ratio of 22:5 omega 3/22:6 omega 3 was higher in affected animals. The relationship of the retinal degeneration to the abnormalities in fatty acid composition in affected animals is not clear, however.
Molecular genetics
The disease in Abyssinian cats has been designated the gene symbol rdAc. Since at least 8 years studies have been in progress to elucidate the gene defect in the disorder. Through candidate gene analysis the following genes have been excluded as causative to rdAc: peripherin / rds [18] and phosducin [19]. The following have also been analyzed without any defect found: rhodopsin, S-antigen, cGMP gated channel beta subunit, transducin subunits alpha, beta and gamma as well as phosphodiesterase, alpha, beta and gamma. The genes for IRBP and phosphokinase C have not yet been excluded, however. Recently a new strategy was initiated, that of homozygocity and comparative mapping approach, in ORDER to find the genetic defect in the disorder. A large number of microsatellites are being analyzed and candidate markers in individual animals will be amplified and further studied.
Physiology of ocular circulation
Under physiologically controlled conditions systemic and ocular circulation was studied in 10 affected and 5 normal control animals using the radioactive microsphere technique [20]. Blood flow determinations were performed under control conditions, after intravenous injection of indomethacin and of L-NNA, an inhibitor of nitric oxide synthase [21, 22]. The local blood flow in retinal and iris tissue was significantly lower in cats affected with advanced disease as compared with the control group, although there was no significant difference in blood flow between the early stage of disease and the control group. Indomethacin had no effect on ocular blood flow in the control GROUP but caused a significant increase in retinal and iridal ocular blood flow at all stages of disease. For L-NNA there was a significant reduction of choroidal blood flow as expected in both affected and controls. These results SHOW that not only retinal blood flow is reduced in affected cats but also iridal blood flow. Cyclooxygenase products with vasoconstrictor activity may partly contribute to the low iridal blood flow.
Electrophysiologic studies of cone function
The use of full-field stimulation and sensitive ERG techniques in our laboratory in Uppsala have enabled us to study animal models electrophysiologically under controlled conditions [23, 24]. By using longer flash stimuli as opposed to Xenon flash stimuli under light adaptation the on- and off- responses of the cone ERG have been investigated [25] in groups of normal cats and cats affected by the early stage of hereditary retinal degeneration (Fig. 2) [26].
The amplitude of the cone b-wave was reduced in affected cats compared to normals and the implicit time of the cone-b-wave was increased at all background levels and at all stimulus durations that gave a discernible b-wave. It has also been shown that by using longer stimuli it is feasable to study a number of reproducible components of the retinal response and specific electrophysiological differences between normal and affected cats can be further evaluated.
Transplantation of neonatal neuroretinal allografts
Photoreceptor transplantation offers the possibility of restoring vision to a degenerate and blind retina. Neuroretinal microaggregates of neonatal tissue were transplanted through a pars plana approach subretinally INTO 12 adult Abyssinian cats with an early stage of photoreceptor degeneration [27, 28]. The surgical procedure was performed under direct visualization and the follow-up recorded using routine clinical methods such as ophthalmoscopy and ERG. Photoreceptors were found in rosettes surrounded by other donor neurons that intermingled intimately with the host retina (Fig. 3).
There was no obvious rejection of donor tissue or other severe complications. Thus, this study showed that the feline neuroretinal transplants survive and develop in the subretinal space of mutant cat retina for at least up to 3 months. | |

Figure 2
Representative cone on-and off- ERG potentials using a full-field, 200 milisec. white light stimulus in the light adapted state. Amplitude and time calibration depicted in the lower left hand corner.
|
|
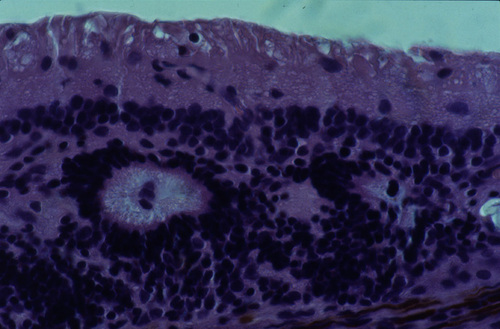
Figure 3
Light micrograph of a 10-week-old transplant in the subretinal space of a 2-year-old recipient Abyssinian cat at the early stage of disease. Rosettes of outer retinal layers are formed and there is evidence of inner and outer segment formation. The section is embedded in paraffin and H&E stained.
|
|
| Acknowledgements | I would like to acknowledge past and present national and international collaborators: Dr. Sven Erik Nilsson, Dept. Ophthalmology, University of Linköping, Dr. Theo van Veen, Dept. Zoophysiology, University of Göteborg, Dr. Berndt Ehinger and Dr. Anitha Bruun, Dept. Ophthalmology, University of Lund, Dr. Siv F. E. Nilsson, Dept. of Physiology and Medical Biophysics, University of Uppsala, Dr. Albert Alm, Dept. Ophthalmology, University of Uppsala, Dr. Björn Ekesten, Dept. Medicine and Surgery, Swedish Univeristy of Agricultural Sciences, Uppsala, and Dr. Lena Ivert, Dept. Ophthalmology, Karolinska Institute, Stockholm, Sweden, Dr. Geoffrey Arden, Institute of Ophthalmology, London, England, Dr. Peter Gouras, Columbia University, New York, Dr. Robert E. Anderson, Cullen Eye Institute, Houston, Dr. Samuel Jacobson, Scheie Eye Institute, Philadelphia, Dr. Michael Gorin, Depts. of Ophthalmology and Human Genetics, Eye and Ear Institute of Pittsburgh, Dr. Marilyn Menotti-Raymond and Dr. Stephen O´Brien, Laboratory of Genomic Diversity, National Cancer Institute, Frederick, Dr. Barbara Wiggert and Dr. Gerald J. Chader, National Eye Institute, National Institutes of Health, Bethesda, U.S.A.
These studies were made possible through grants obtained FROM the Foundation Fighting Blindness (U.S.A.), the Swedish Medical Research Council project no.: 19X-09938 and FROM the Foundation for Comparative Pathology and Professor Sten-Erik Olsson, Sweden. | | | References | 1. Narfström, K., Retinal degeneration in a strain of Abyssinian cats: A hereditary, clinical, electrophysiological and morphological study. Linköping University Medical Dissertations No. 208 Linköping (1985)
2. Narfström, K., Arden, G.B. and Nilsson, S.E., Retinal sensitivity in hereditary retinal degeneration in Abyssinian cats: Electrophysiological similarities between human and cat. Br J Ophthalmol 73:516-521 (1989)
3. Narfström, K., Progressive retinal atrophy in the Abyssinian cat: Clinical characteristics. Invest Ophthalmol Vis Sci.26:193-200 (1985)
4. Narfström, K., Nilsson, S.E. and Andersson, B.E., Progressive retinal atrophy in the Abyssinian cat. Studies of the DC-recorded electroretinogram and the standing potential of the eye. Br J Ophthalmol 69:618-623 (1985)
5. Narfström, K. and Nilsson, S.E., Progressive retinal atrophy in the Abyssinian cat: Electron microscopy. Invest Ophthalmol Vis Sci 27:1569 (1986)
6. Narfström, K., Hereditary progressive retinal atrophy in the Abyssinian cat. J of Hered 74:273-276 (1983)
7. Narfström, K., Wilén, M. and Andersson, B.E., Hereditary retinal degeneration in the Abyssinian cat: Developmental studies using clinical electroretinography. Docum Ophthalmol 69:111-118 (1988)
8. Narfström, K. and Nilsson, S.E., Morphological findings during retinal development and maturation in hereditary rod-cone degeneration in Abyssinian cats. Exp Eye Res 49:611-628 (1989)
9. Vogel, M., Postnatal development of the cat´s retina: A concept of maturation obtained by qualitative and quantitative examinations. Albrecht von Greafes Arch Klin Ophthalmol 208: 93-107 (1978)
10. Ehinger, B., Narfström, K., Nilsson, S.E. and van Veen, T., Photoreceptor degeneration and loss of immunoreactive GABA in the Abyssinian cat retina. Exp Eye Res 52:17-25 (1991)
11. Ekström, P., Sanyal, S., Narfström, K., Chader, J. and van Veen, T., Accumulation of glial fibrillary acidic protein in Muller radial glia during retinal degeneration. Invest Ophthalmol Vis Sci 29:1363-1371 (1988)
12. Narfström, K., Nilsson, S.E., Wiggert, B., Lee, L., Chader, J. and van Veen, T., Reduced IRBP level, a possible cause for retinal degeneration in the Abyssinian cat. Cell and Tissue Res 257:631-639 (1989)
13. Meeks, R.G., Zaharewitz, D., Chen, F., Membrane effects of retinoids: possible correlation with toxicity. Arch Biochem Biophys 207: 141-147 (1981)
14. Wiggert, B., van Veen, T., Kutty, G., Lee,L., Nickerson, J., Si, J-S., Nilsson, S.E.G., Chader, G.J. and Narfström, K., An early decrease in interphotoreceptor retinoid-binding protein gene expression in Abyssinian cats homozygous for hereditary rod-cone degeneration. Cell and Tissue Res 278:291-298 (1994)
15. Jacobson, S.G., Kemp, C.M., Narfström, K. and Nilsson, S.E., Rhodopsin levels and rod-mediated function in Abyssinian cats with hereditary retinal degeneration. Exp Eye Res 49:843-852 (1989)
16. Converse, C.A., Hammer, H.M., Packard, C.J., Shepherd, J., Plasma lipid abnormalities in retinitis pigmentosa and related conditions. Trans Ophthalmol Soc U.K. 103: 508 (1983)
17. Anderson, R.E., Maude, M.B., Nilsson, S.E. and Narfström, K., Plasma lipid abnormalities in the Abyssinian cat with a hereditary rod-cone degeneration. Exp Eye Res 53:415-417 (1991)
18. Gorin, M.B., Snyder, S., Narfström, K. and Curtis, R., The cat rds transcript: candidate gene analysis and phylogenic sequence analysis. Mammalian genome 4:544-548 (1993)
19. Gorin, M.B., To, A. and Narfström, K., Sequence analysis and exclusion of phosducin as the gene for the recessive retinal degeneration of the Abyssinian cat. Biochemica et Biophysica Acta, 1260: 323-327 (1995)
20. Alm, A., Bill, A., The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arteriolar carbon dioxide tension on uveal and retinal blood flow in cats. Acta Physiol Scand 84: 306-319 (1972)
21. Narfström, K., Nilsson, S.F.E., Mäepea, O., Alm, A., Ocular blood flow is decreased in Abyssinian cats with hereditary retinal degeneration. Invest Ophthalmol Vis Sci 38: S440 (1997)
22. Nilsson, S.F.E., Mäepea, O., Alm, A., Narfström, K., Ocular blood flow and retinal metabolism in Abyssinian cats with hereditary retinal degeneration. Abstract, Eric K. Fernström Symposium, Lund (1997)
23. Narfström, K., Andersson, B.-E., Andreasson, S. and Gouras, P., Clinical electroretinography in the dog using Ganzfeld stimulation: A practical method of examining rod and cone function. Documenta Ophthalmologica 90:279-290 (1995)
24. Narfström, K., Ivert, L., Yamamoto, S. and Gouras, P., Adaptation of rod and cone electroretinograms in the Abyssinian cat hereditary rod-cone degeneration. Clin Vision Sci. 8:177-185 (1993)
25. Sieving, P.A., Photopic on- and off-pathways abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc 91: 701-773 (1993)
26. Ekesten, B., Andersson, B.E., Narfström, K., On- and off-responses in the cone-mediated ERG in normal and dystrophic cats. Abstract, International Society for Clinical Electrophysiology of Vision, Asilomar, California (1997)
27. Narfström, K., Ivert, L., Naeser, P., Gouras, P., Transplantation of neonatal neural retina in photoreceptor degeneration of cats. In press in Degenerative retinal diseases. ed.: LaVail, Hollyfield and Anderson, CRC Press. (1997)
28. Ivert, L., Narfström, K. and Gouras, P., Photoreceptor transplants in a feline model of RP. Invest Ophthalmol Vis Sci 38: S260 (1997). | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in