|
|
 |
 |
 |
 |
|
|
Model for Night Blindness
Digital Journal of Ophthalmology 1998
Volume 4, Number 4
September 1, 1998
|
Printer Friendly
|


 Yozo Miyake, M.D.
Yozo Miyake, M.D. | Nagoya University School of Medicine Masayuki Horiguchi,M.D. | Nagoya University School of Medicine Satoshi Suzuki, M.D. | Nagoya University School of Medicine Mineo Kondo, M.D. | Nagoya University School of Medicine Atsutoshi Tanikawa, M.D. | Nagoya University School of Medicine Hiroko Terasaki, M.D. | Nagoya University School of Medicine
|
|
|
| Abstract | Keywords
Congenital stationary night blindness, ERG, human, EOG, retina, retinal degeneration, retinitis pigmentosa, transplantation | | | Introduction | Electrophysiological analysis of variable congenital stationary night blindness (CSNB) is important, because it can provide informations about the locus for night blindness as well as the abnormality of cone visual pathway, if any, in the retina.
The Schubert-Bornschein type CSNB [1] shows an essentially normal fundus and negative electroretinogram (ERG) which indicates the normal a-wave with extremely reduced b-wave. We previously reported [2] that CSNB with negative ERG can be divided INTO two types, complete and incomplete, based on the difference in rod visual functions, cone mediated ERGs, ERG oscillatory potentials, degree of refractive errors, and family survey. It has been concluded that complete and incomplete CSNB are different clinical entities [2,3].
Oguchi's disease, first described by Oguchi in 1907 [4], is an unusual form of CSNB characterized by a peculiar gray-white discoloration of the fundus. Carr and Gouras [5] and Carr and Ripps [6] speculated that in Oguchi's disease the defect is in the post-receptor processing of rod-mediated signals according to their results of ERG, electrooculogram (EOG) and retinal densitometry.
We compared the patients with complete CSNB, incomplete CSNB, and Oguchi's disease in terms of ERG and EOG and found that each CSNB has a different pathogenesis in both rod and cone visual pathways and may provide a model concept for night blindness. | | | Materials and Methods | 1. Full-field rod and cone ERGs
Conventional full-field ERGs were recorded after 30 minutes of dark adaptation. The rod (scotopic) ERG was recorded with a blue light at an intensity of 5.2x10-3 cd/m2. sec. The cone (photopic) ERG and 30-Hz flicker ERG were recorded with a white stimulus intensity of 4 cd/m2. sec and ??? 0.9 cd/m2. sec, respectively. Figure 1 shows examples of rod, cone, and 30-Hz flicker ERG in three kinds of CSNB. As previously reported [2], a complete CSNB disclosed nonrecordable rod ERG with relatively restored cone and 30-Hz flicker ERG, whereas an incomplete CSNB demonstrated subnormal rod ERG with deteriorated cone ERG. An Oguchi's disease patient showed nonrecordable rod ERG after 30 minutes of dark adaptation and normal cone and 30-Hz flicker ERG. We examined 7 patients with Oguchi's disease and all patients showed similar results [7].
The rod-cone mixed ERG was recorded with a white intense stimulus of 44.2 cd/m2. sec. Figure 2 shows ERGs of a normal subject, a complete and an incomplete CSNB patient, and 7 patients with Oguchi's disease recorded after 30 minutes of dark adaptation. In addition, ERGs of 6 of the 7 patients with Oguchi's disease recorded after 3 to 6 hours of dark adaptation are also shown. The complete and the incomplete CSNB had a normal a-wave and a reduced b-wave (smaller than b-wave), showing a negative configuration. The oscillatory potentials are better recordable in the incomplete CSNB. These findings were observed in most patients with complete and incomplete CSNB as previously reported [2]. In 7 patients with Oguchi's disease [7], the ERG showed a negative configuration. However, the amplitude of the a-wave ranged FROM 116 to 168 uV (mean:13 uV), approximately one-half or one-third of the normal ( 310 ±94 uV; mean±SD); the b-wave was nearly absent, but the oscillatory potentials were clearly seen in all patients. Following 3 to 6 hours of dark adaptation, 5 of 6 patients showed increased amplitude (particularly in the a-wave) but one (case 6) showed no significant increase even after 3 hours of dark adaptation [7].
2. ERG spectral characteristics
To determine whether the dark-adapted ERG responses of Oguchi's disease shown in Figure 2 represented rod or cone activity, we examined the spectral characteristics. ERGs were recorded after 30 minutes of dark adaptation and 10 minutes of light adaptation with a white background light of 40 cd/m2 and responses were recorded with short-(B), middle-(G) and long-(R) wavelength stimuli, which were photopically equivalent. In Figure 3, the ERG waveforms obtained in response to the photopically matched flashes were quite similar in a given condition, indicating that both dark-adapted and light-adapted responses reflect cone activity. Identical procedures were used for two additional patients; the results were essentially identical.
3. EOG
The EOG was recorded in total darkness at one minute intervals for 15 minutes and then under light adaptation (262 cd/m2) for the next 15 minutes. The normal L/D ratio in our laboratory ranges FROM 1.71 to 2.94. EOG of 17 patients with complete CSNB, 13 patients with incomplete CSNB, and 6 patients with Oguchi's disease were compared with 49 normal subjects in Figure 4. The EOGs of complete and incomplete CSNB patients were nearly within normal range, whereas those of Oguchi's disease were abnormal.
4. Photopic long-flash ERG
The rectangular monochromatic repetitive stimuli under photopic condition (photopic long-flash ERG) was used to isolate the ON- and OFF-responses in the cone visual pathway. The colored-light stimuli, produced by a 560 nm monochromatic filter using a 500 W xenon arc, was passed in front of the eye and the white background illumination was led INTO the fiber optics mounted in a Ganzfeld system. The intensities of colored stimulus and white background illumination were 870 mW/m2 and 130 mW/m2, respectively. The stimulus frequency of the rectangular stimuli was 4-Hz with 125 msec of light on and 125 msec light off; 32 responses were averaged by a signal processor.
Figure 5 shows photopic long-flash ERGs in a normal subject, 4 complete CSNB patients, 4 incomplete CSNB patients, and 3 Oguchi's disease patients. The complete CSNB patients showed a normal a-wave, small b-wave, and large off- (d) wave. On the contrary, the incomplete CSNB patients disclosed a small a-wave, relatively large b-wave, and small OFF- (d) wave. The Oguchi's disease patients showed a normal photopic long-flash ERG in both ON- and OFF-component. | |

Figure 1
Full-field scotopic (rod), photopic (cone) and 30-Hz flicker ERG in a normal subject and patients with three kinds of night blindness.
|
|
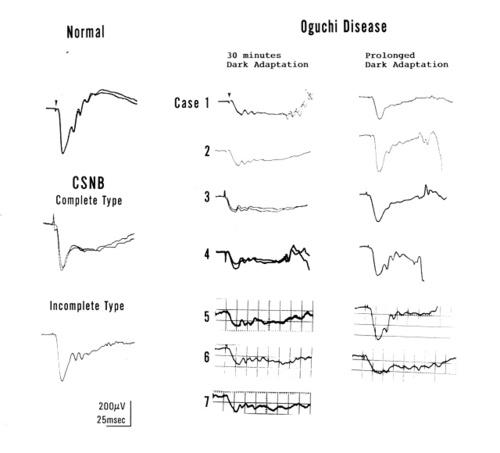
Figure 2
Rod-cone mixed ERG recorded with a white bright light after 30 minutes of dark adaptation in a normal subject, a patient with complete and incomplete CSNB, and 7 patients with Oguchi's disease. ERGs of 6 of 7 patients with Oguchi's diseases recorded after 3 to 6 hours (prolonged) dark adaptation are shown in the right
|
|
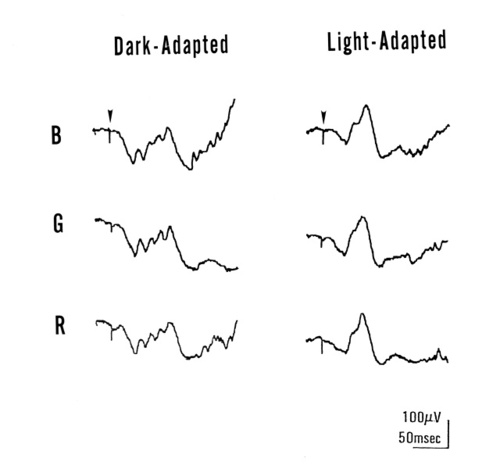
Figure 3
ERGs obtained FROM a patient with Oguchi's disease after 30 minutes of dark adaptation (left) or 10 minutes of light adaptation (right). Responses were recorded with short-wavelength (S) flash, middle-wavelength (M) flash, and long-wavlength (L) flash of equivalent photopic intensity.
|
|

Figure 4
Comparison of EOG ratio between normal subjects, complete CSNB, incomplete CSNB, and Oguchi's disease patients
|
|

Figure 5
Photopic long-flash ERGs in a normal subject, complete CSNB, incomplete CSNB, and Oguchi's disease patients
|
|
| Results | The electrophysiological analysis was made in patients with complete type, incomplete type CSNB, and Oguchi's disease. The different pathogenesis was clearly observed in rod a-wave and long-flash photopic ERG. After 30 minutes of dark adaptation, the rod a-wave was absent in Oguchi's disease, whereas it was normally recordable in complete and incomplete CSNB. This results suggest that the locus of night blindness may lie in the rod itself in Oguchi's disease, while it may be in the post-rceptoral processing of rod visual pathway in complete and incomplete CSNB. The photopic long-flash ERG revealed off dominated response in complete CSNB, on dominated response in incomplete CSNB, and normal response in Oguchi's disease.
Above results indicate that these three kinds of stationary night blindness have a different pathogenesis in both rod and cone visual pathways. | | | Discussion | In both complete and incomplete CSNB, the amplitude of a-wave in mixed rod-cone ERG is normal, indicating that the rod a-wave is essentially normal. It is thus conceivable that the defect of rod visual pathway is likely to be caused by the post-receptoral processing of rod-mediated signals as was suggested by others [5,6]. The normal EOG also supports this hypothesis [5,6]. We reported previously [9] that neither the rod b-wave nor the scotopic threshold response (STR) was recordable in complete CSNB, suggesting that the rod signal cannot reach the rod bipolar cells because of the defect of ON-synapse to the rod bipolar cells. We also found [9] that the near-threshold b-wave of incomplete CSNB was normal, although the rod ERG b-wave in routine recording (brighter than the stimulus for near-threshold b-wave) was subnormal. Since the STR of incomplete CSNB was abnormal, we hypothesized that the defect of the rod visual pathway in incomplete CSNB may be proximal to the rod bipolar cells [9].
In 1965, Carr and Gouras [5] reported detailed ERG findings of 4 Caucasian patients with Oguchi's disease. The patients' ERGs when recorded with a relatively intense stimulus after 10 minutes of dark adaptation, showed a negative form with a normal a-wave and a small b-wave, similar to that of complete CSNB. In a later study, Carr and Ripps restudied one of these patients and found a normal EOG and normal concentrations and kinetics of rod visual pigments. These authors concluded that a defect in the post-receptor signals is the cause of night blindness [5,6].
In our patients, however, the a-wave amplitude of the mixed rod-cone ERG was significantly lower than normal or complete and incomplete CSNB when recorded after 30 minutes of dark adaptation. In addition, our analysis using ERG spectral characteristics indicated that the rod a-wave is absent in Oguchi's disease after 30 minutes of dark adaptation. The absence of rod a-wave, with abnormal EOG, strongly suggests that the rod itself is abnormal in our Oguchi's disease patients. These electrophysiological findings seem to be consistent with the recent molecular findings of Oguchi's disease. Fuchs and associates [10] reported the functional defect of arrestin, the phototransduction of rod, in Japanese patients with Oguchi's disease. More recently, Yamamoto and associates [11] found defects in the rhodopsin kinase gene in two Caucasian patients with Oguchi's disease. Since no retinal densitometry has been done in our patients, the concentration of rhodopsin and the kinetics are unknown. If rod densitometry is normal, the defect lie in the rod phototransduction (functional defect of arrestin) as was shown by Fuchs and associates [10]. In case it is abnormal, the defect would involve the rhodopsin pigments just as shown by Yamamoto and associates [11]. In either case, our results strongly suggest that the majority of Oguchi's patients have some abnormality in the rods itself.
The second concern is the abnormality of the ON/OFF pathways of the cone visual system. Evaluation of the ON- and OFF-responses in the cone mediated ERG has opened a new door for identifying the pathology of depolarizing (ON) and hyperpolarizing (OFF) bipolar cells since a pharmacological approach can selectively block either ON or the OFF pathway [12,13]. Our results suggested that complete CSNB is resulted FROM a dysfunction of the sign-inverting synapse of both rod and cone depolarizing bipolar cells (ON pathway). The photopic long-flash ERG in complete CSNB was similar to that recorded in a monkey after blocking the sign-inverting synapse with APB (2-amino 4 phosphonobutyric acid) [14,15]. On the other hand, the wave shape of incomplete CSNB was similar to that of monkey ERG treated by KYN (kynurenic acid) to block the OFF synapse to the cone bipolar cells [14,15]. These results suggest that complete and incomplete CSNB have a defect of ON- and OFF-synapse to the cone bipolar cells, respectively.
Unlike the selective dysfunction of ON- or OFF-system in complete or incomplete CSNB, Oguchi's disease showed normal ON- and OFF-system in cone visual pathway. It is interesting that there is a fundamental difference in the cone visual system between three kinds of CSNB, which may be related, for example, to the visual acuity. The visual acuity in Oguchi's disease is normal, while it is moderately low in both complete and incomplete CSNB. It would be worthwhile to examine the differences in psychophysical visual functions in response to the ON- and OFF-visual pathway in these three kinds of CSNB.
In combination of rod and cone visual pathway, the complete CSNB is likely to have a defect of ON-synapse to both rod and cone bipolar cells, and only the OFF visual pathway is adequately functioning (model of OFF-retina). The incomplete CSNB, on the other hand, has a selective defect of the OFF-synapse to cone bipolar cells. Since the defect of rod vision may lie proximal to rod bipolar cells [9], ON-synapses to both cone and rod bipolar cells may function well (ON-retina). Oguchi's disease has a defect in the rod itself. The ON-synapse to the rod bipolar cells, the ON- and OFF-synapses to the cone bipolar cells may function normally (model of rod defect). | | | References | 1) Schubert, G., Bornschein, H. Beitrag zur Analyse des menschlichen Electroretinogramms. Ophthalmologica 123:396-413 (1952)
2) Miyake, Y., Yagasaki, K., Horiguchi, M., Kanda, H., Kawase, Y. Congenital stationary night blindnes with negative electroretinogram. A new classification. Arch Ophthalmol 104:1013-1020 (1986)
3) Miyake, Y., Horiguchi, M., Ota, I., Shiroyama, N. Characteristic ERG flicker anomaly in incomplete congenital stationary night blindness. Invest Ophthalmol Vis Sci 28:1816-1823 (1987)
4) Oguchi, C. Uber eine Abart von Hemeralopie. Acta Soc Ophthalmol Jpn 11:123-134 (1970)
5) Carr, R.E., Gouras, P. Oguchi's disease. Arch Ophthalmol 73:646-656 (1965)
6) Carr, R.E., Ripps, H. Rhodopsin kinetics and rod adaptation in Oguchi's disease. Invest Ophthalmol 6:426-436 (1967)
7) Miyake, Y., Horiguchi, M., Suzuki, S., Kondo, M., Tanikawa, A. Electrophysiological findings in patients with Oguchi's disease. Jpn J Ophthalmol 40:511-519 (1996)
8) Miyake, Y., Yagasaki, K., Horiguchi, M., Kawase, Y. On- and off- responses in photopic electroretinogram in complete and incomplete types of congenital stationary night blindness. Jpn J Ophthalmol 31:81-87 (1987)
9) Miyake, Y., Horiguchi, M., Terasaki, H., Kondo, M. Scotopic threshold response in complete and incomplete types of congenital stationary night blindness. Invest Ophthalmol Vis Sci 35:3770-3775 (1994)
10) Fuch, S., Nakazawa, M., Tamai, M., Oguchi, Y., Gal, A. A monozygous 1-base pair deletion in the gene is a frequent cause of Oguchi's disease in Japanese. Nature Genet 10:360- 362 (1995)
11) Yamamoto, S., Sippel, K.C., Berson, E.L., Dryja, T.P. Defects in the rhodopsin kinase gene in the Oguchi's form of stationary night blindness. Nature Genet 15:175-178 (1997)
12) Slaughter, M.M., Miller, R.F. 2-amino-phosphobutric acid: A new pharmacological tool, for retinal research. Science 211:182-185 (1981)
13) Slaughter, M.M., Miller, R.F. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature 303:537-538 (1983)
14) Sieving, P.A. Photopic on- and off pathway abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc 91:701-773 (1993)
15) Sieving, P.A., Murayama, K., Naarendrop, F. Push-pull model of the primate photopic electroretinogram: A role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519-532 (1994). | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in