|
|
 |
 |
 |
 |
|
|
Human Neural Retinal Transplants INTO Retinitis Pigmentosa: Facts and Controversy
Digital Journal of Ophthalmology 1998
Volume 4, Number 5
September 15, 1998
|
Printer Friendly
|


 Manuel del Cerro, M.D.
Manuel del Cerro, M.D. | University of Rochester School of Medicine David A. DiLoreto, Jr., M.D., Ph.D. | University of Rochester School of Medicine Constancia del Cerro, M.S. | University of Rochester School of Medicine Eliot S. Lazar, M.D. | University of Rochester School of Medicine
|
|
|
| Abstract | Objective
To discuss the scientific facts and controversies surrounding human retinal transplantation.
Methods
In January 1995, we began multicenter, phase I clinical studies to assess the safety of transplanting neural retina INTO the subretinal space of patients with advanced retinal degenerative disease. A total of 18 patients with retinitis pigmentosa and one patient with age-related macular degeneration were transplanted with a suspension of fetal neural retina (14 to 18 weeks gestational age). No patients received systemic immunosuppression. Patients underwent pre-operative and post-operative visual testing, that in the most recent cases included a newly developed test battery for low level vision, assessment of accurate projection of rays, visual acuity, ERG, and visual fields.
Results
The longest follow-up to date has been 2.5 years on four patients. No evidence of inflammation, infection, overt rejection or compromise to the host eye was noted in any patients. There was one case of retinal detachment. In this patient, vision worsened FROM hand motion to light perception. Three patients showed a 4 to 10 fold increase in light sensitivity starting one to two months after transplantation. Two of these patients have since returned to their pre-operative baseline. Six patients have shown improvement in vision starting 4 to 6 months post-transplantation which has been sustained to date. Two of these patients developed the ability to undergo visual field testing.
Conclusion
Safe and efficient delivery of neural retinal cells INTO the subretinal space of patients with advanced retinitis pigmentosa has been achieved. The results warrant further studies to quantify the possible benefits of neural retinal transplantation in patients suffering FROM retinal degenerative diseases. A variety of issues regarding human retinal transplantation is discussed.
Keywords
age-related macular degeneration, fetal cells, human, photoreceptors, retina, retinal degeneration, retinitis pigmentosa, transplantation | | | Introduction | After twelve years of laboratory research [1,2], clinical studies of neural retinal was started in early 1995. On January 18 of that year, a surgical team headed by Eugene de Juan, Jr., in collaboration with members of the Rochester Retinal Transplantation GROUP headed by Manuel del Cerro, grafted a suspension of fetal neural retinal cells INTO one eye of a patient affected by age-related macular degeneration (AMD) [3]. The first transplantation INTO a patient with retinitis pigmentosa (RP) followed one month later. On February 21, 1995, a surgical team directed by Taraprasad Das and combined efforts with del Cerroís research team to transplant a suspension of fetal neural retinal cells INTO the subretinal space of a patient affected by advanced RP [4,5].
These operations were the culmination of months of surgical planning and of extensive laboratory research. Since then, 18 RP patients have been transplanted with human fetal neural retinal cells by our collaborators both in the USA and India. Although the primary objective was to determine the safety of the procedure, changes in the quality of vision have been observed in several of these patients. The safety of the procedure has been proven, giving an incentive to continue these studies searching for improved parameters for the operation. Although, initial results offer at least a hope to blind RP patients, it needs to be clearly understood that the procedure is still experimental and that the very concept is not without critics. Here we propose to review the clinical facts as they have developed up to the present and the controversy surrounding human retinal transplantation. | | | Materials and Methods | The same basic transplantation procedures have been used at the Wilmer Eye Institute, Baltimore, Maryland, USA, and the L.V. Prasad Eye Institute, Hyderabad, India, with exceptions noted below.
Donor Tissue
The donor tissue was human fetal neural retina obtained with informed, written consent FROM elective abortions (14 to 18 weeks gestational age) following federal and institutional guidelines. The blood of the women donors was tested for the presence of transmissible pathogens. In all cases the donors were negative for all of the tested agents.
The fetal eyes were dissected using aseptic technique. The enucleated eyes were held in ice-cold Optisol (Chiron Vision, Irvine, CA) and dissected to obtain a pure sheet of neural retina within 3 hours of obtaining the eyes [6]. The neural retina was transferred INTO Dulbeccoís Modified Eagles Medium (DMEM), low glucose (Gibco) at 4 C. A small portion of the neural retina was separated using Vannas scissors. This fragment was used for the trypan blue viability test as previously described [6]. Only tissue that had a viability of greater than 80% was used. The remaining portion of the neural retina was mechanically dissociated in DMEM using Vannas scissors. The resulting suspension of single cells and small cell clusters was loaded INTO a two-function tissue manipulator (Grieshaber, Kennesaw, Georgia) just prior to transplantation. Prior to transplantation, all tissue was stored at 4 C for less than 24 hours at the Prasad Institute and less than 72 hours at the Wilmer Institute.
Patients
The study was reviewed and approved by the ethics committees and institutional review boards of the Prasad Institute, the Wilmer Institute, and the University of Rochester School of Medicine and Dentistry. The tenets of the Helsinki convention were followed strictly. Patients selected for this study carried the diagnosis of RP (and AMD) established FROM patient history, clinical examination, and clinical tests.
Prior to transplantation, all patients were screened to rule out transmissible diseases including HIV, hepatitis B, syphilis, etc. In addition, all patients (n=19) selected for surgery met the following criteria: (1) clinical condition: bilateral expression of RP (with the one exception of the AMD patient); (2) age: 21 to 70 years of age (with the one exception of the AMD patient who was 95 years old); (3) visual acuity, light perception or hand motion (except for a patient counting fingers at not more than 15 cm in the operated eye); (4) optic disc: no clinically detectable atrophy; (5) macula: no detectable gross changes either clinically or on fluorescein angiography (with the one exception of the AMD patient); and (6) informed consent to participate in the study. The worse eye, as subjectively felt by the patient and confirmed by pre-operative examination (see below), was chosen for surgery.
Visual Examination
Seven patients at Wilmer underwent a newly designed test battery to monitor visual function in patients with extremely attenuated vision as previously described [7]. Twelve patients at Prasad underwent visual screening twice on two separate dates prior to transplantation and multiple times post-transplantation that included visual acuity testing, full field ERG [8], and visual field testing [9]. All patients underwent fluorescein angiography before and after transplantation.
Surgical Procedure
A standard three port vitrectomy was performed, a small retinotomy was made, and a two function tissue manipulator (Grieshaber) loaded with the retinal cell suspension was introduced through the retinotomy INTO the subretinal space (Figure 1). The neural retinal cells were injected INTO the subretinal space slowly and under continuos visualization. The total volume injected did not exceed 150 µl in any patient.
Following transplantation at the Prasad Institute, barrage endophotocoagulation was performed at the site of the retinotomy and also around to the graft. No endophotocoagulation was used at Wilmer. Non-steroidal anti-inflammatory drugs and topical steroids were administered during the immediate post-surgical period. No systemic immunosuppressive agents or corticosteroids were used pre- or post-operatively. | |
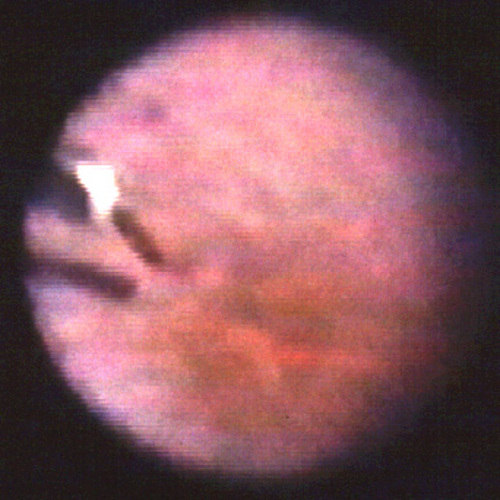
Figure 1
Video frame showing the tip of the two function manipulator overlying the retina, immediately preceding the insertion of the tip INTO the retinotomy.
|
|
| Results | The follow up of the 19 patients transplanted up to this time has ranged FROM 9 to 30 months.
The first goal of the study was to determine the short- and long-term safety of the grafting procedure. The combined results of the two surgical series confirm the safety of transplantation.
Complications were few and are detailed below. Full descriptions of the two studies will be published independently in the peer-reviewed scientific literature. What follows is a brief summary. Its purpose is to serve as a platform to discuss the controversial issues surrounding human retinal transplantation.
The L.V. Prasad Series
Table 1 summarizes the pre- and post-operative visual acuity of the eye that was transplanted.
Preoperatively, the fundi of all patients showed marginal pallor of the optic discs in both eyes, narrow arterioles and pigmented bone spicule formation along the vessels (Figure 2). No macular edema was detected on fluorescein angiography. The electroretinograms and visual evoked potentials were indistinguishable FROM noise. Visual field examination was impossible to carry out in any patient prior to surgery.
One patient developed a retinal detachment immediately after surgery and visual acuity changed FROM hand motion to light perception. This patient refused surgery to REPAIR the detachment. Two patients developed microhemorrhages close to the retinotomy site at the time of surgery that disappeared within two weeks. As shown in TABLE 1, six patients showed improvement beginning at four to six months post-transplantation in all cases. Two of the six patients that showed improvement developed the ability to take a visual field test at 6 months of post-transplantation. There was no change in full field electroretinogram in any of the patients. The visual acuity and visual field improvements have not regressed in any patients. There were no changes in the fellow eyes in any of the patients at any time.
The Wilmer Series
No adverse effects were found in any patient. No patient developed any complications. No evidence of acute immunologic reaction or rejection was seen. One patient developed a focal mild vascular leakage over the area of transplant at one year. By two years the transplants could no longer be seen. Three of the seven patients had a sustained repeatable 4 to 10 fold increase in light sensitivity. The increase occurred within 1 to 2 months post-transplantation. One patient regained limited "form" vision (large moving high contrast objects). Increased projection was noted in 3 patients. No patient developed Snellen acuity. No changes occurred in the ERG or SLO. | |
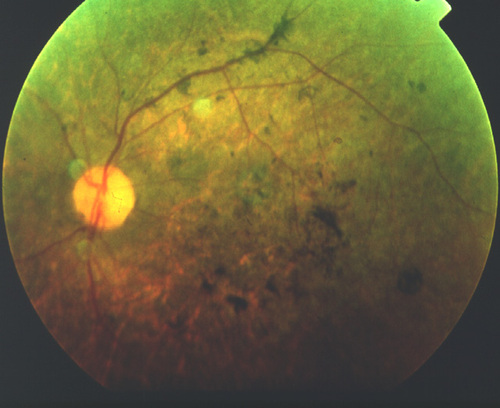
Figure 2
Pre-operative fundus view of a patient in the Hyderabad series showing typical, advance RP changes.
|
|
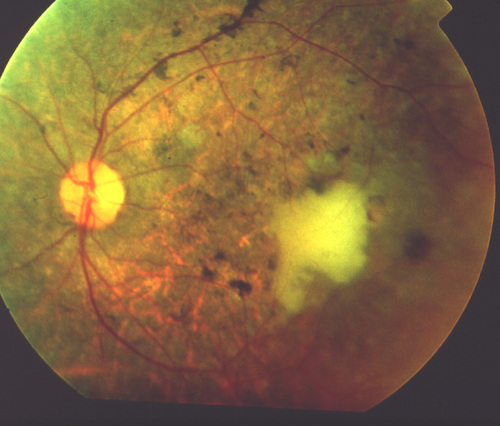
Figure 3
Post-operative fundus view at day 1, of the same patient shown in Figure 2. A large, irregular cell clump is visualized as a whitish mass at a parafoveal location.
|
|
| Discussion | Human retinal transplantation is in its beginnings. Its implementation has nonetheless created considerable, sometimes excessive expectations and paradoxically, some extremely negative reactions. A discussion of our human transplantation experience as well as some controversial issues in human retinal transplantation are addressed below.
Is human retinal transplantation necessary?
A variety of therapies have been tried for the treatment of RP without proven effect in slowing or reversing the progression of the disease [10]. Administration of vitamin A has been shown to delay the deterioration of the electroretinogram [11]; however this treatment has not gained total acceptance [10,12,13]. Also, the action of A vitamin is one retarding the progress of the disease rather than of reversing its effects. In advanced stages of RP when the vision is reduced to light perception, the photoreceptor cell population is significantly reduced [14-18], a situation presently irreparable by pharmacological intervention. Since RP is a disease of rods and cones [14-18] and the retinal pigment epithelium is relatively spared [19], a logical strategy in advanced cases of RP is the replacement of healthy photoreceptors by means of grafting.
Is human retinal transplantation premature?
This has been an often asked question. Today, however, the question is moot, since human retinal transplantation (both RPE and neural retina) has already begun in multiple centers around the world including USA, Europe, and Asia. Many more centers are ready to join in the near future.
Our results have demonstrated that human fetal neural cell transplantation in RP is a safe procedure, as none of the patients experienced overt inflammation, infection, or clinically observable rejection of the graft during an extended period of follow-up. Induction of neovascularization as a result of putative growth factors derived FROM the grafted fetal cells was a concern to some critics, in practice it never developed.
Moreover, laboratory research in this field has been ongoing since the early 1980ís. Studies of retinal transplantation INTO animal eyes has shown that the fetal or perinatal neural retina can provide new photoreceptors to the damaged host retina [20-22]. A functional improvement may be the outcome of these neural retinal grafts [23]. Cell transplantation, whether to treat Parkinsonís disease, diabetes, or RP is a novel and indeed revolutionary therapy. If effective, any of these procedures will represent a medical breakthrough. By definition, medical breakthroughs are ahead of contemporary treatment of a disease, if any exists at all. It is in this regard only that human retinal transplantation could be considered premature.
Should more laboratory research be conducted on retinal transplantation?
The answer to this question is unequivocal. Much more experimental laboratory work needs to be performed to answer old and new questions pertaining to retinal transplantation. In this regard, retinal transplantation is on an equal footing with bone marrow, liver, and corneal transplantation. In each of those cases additional information still needs to be gathered to increase the efficacy of the grafts and their survival rates. It is encouraging to note, however, that the implementation of human retinal transplantation has stimulated a recent, sharp increase in experimental work. Not only has animal work increased dramatically in the last two years, but in vitro work [24] and post-mortem histopathological studies are now conducted [25,26] predicated on their ultimate relevance to human transplantation.
What is the quantitative, objective DATA in those patients who reported improvement in vision?
In this, the first attempt to transplant neural retinal cell INTO RP patients, it was necessary to assess the surgical feasibility and the safety of the procedure. Indeed, the technique described is simple and reproducible. The placement of fetal neural retinal cells in the subretinal space of patients with advanced RP was without any surgically or clinically related complications in any of the patients. The subjective improvement in vision reported by some patients, while exciting, is by no means definite proof of a beneficial effect. This study should be followed by work specifically designed to objectively assess the effects of neural retinal cell transplantation in patients with retinal degenerative diseases. Fortunately new and better methods are available for the quantitative evaluation of low vision [7]. These methods are now been used in both of our collaborative studies [3, T. Das, personal communication].
How can spontaneous changes and/or the placebo effect be ruled out?
The study was the first of its kind and was therefore designed to determine if transplantation is a feasible treatment option. In phase I clinical trials, the safety of the intervention is the prime consideration. The patients in this initial series had little remaining functional vision pre-operatively, so that any surgically related complication would have minimal detrimental effect.
Six patients that showed improvement did so in a delayed (four to six months post-transplantation) and sustained (no deterioration after improvement) manner. Also, in these patients no improvement was found in the un-operated eye. If changes were due to a placebo effect, classically the patient would report improvement immediately following treatment and this improvement would gradually deteriorate to baseline with time.
In three patients that showed improvement, their improvement was noted more immediately (one to two months post-transplantation), changes were also noted in the un-operated eye and two of these patients since reported decline in vision. Certainly, spontaneous fluctuations in the patients disease or the placebo effect may have been acting in these patients.
Thus, in further studies, designed to test the efficacy of the transplantation objective, quantitative DATA will need to be gathered using protocols that have just been reported and implemented [7]. Pre-operative intervisit variation in vision needs to be determined. Future VEP and ERG testing will need to be adjusted by using sufficient averaging and light intensities to maximize the possibility of detecting any changes positive or negative. It will also be necessary to document retinal location of the visual improvement and correlate that to the area of transplantation.
In ORDER to perform a phase II clinical trial to test efficacy, the effect of the transplant surgery itself or the "sham" effect needs to be controlled for. Indeed, recent DATA SHOW upregulation of growth factors within the retina after mechanical injury [27,28]. Theoretically, such an effect could provide temporary stabilization or improvement of disease in RP patients. The effect could also have a positive influence on the grafted cells, providing an environment rich in factors that promote survival and differentiation.
If improvement can be confirmed, what is the mechanism of visual recovery?
Until histopathological information becomes available, the exact mechanism of any documented visual improvement FROM neural retinal transplantation studies will be difficult to determine. Animal studies SHOW development of synaptic connections after neural retinal transplantation [20-22]. If synaptic connections develop between the grafted cells and the host, then the grafted cells have at least the potential to restore function [23]. Circuit restoration through transplantation may be possible, in principle, because direct electrical stimulation of the retina proves the survival of functional retinal circuitry in RP patients [29]. The existence of plasticity in the adult human retina even in the presence of extensive pathology has been elegantly demonstrated [30].
Since it is not yet known if synaptic connections are established after human neural retinal transplantation, alternative mechanisms must be considered. Growth factors rescue photoreceptor cells in animals affected by inherited retinal degeneration [31] and stimulate photoreceptor differentiation [32]. Trophic influences FROM the grafted fetal retinal cells may have bolstered the functionality of the hostís presumably limited photoreceptor cell population. Such an effect should express itself more acutely; this has been recently reported in one of our collaborative studies [3]. However, if the time period for the improvement to become detectable appears after several months, this interval would be more congruent with the time required for cell differentiation and synapse formation. Until additional DATA can be collected, this issue remains open.
Is immunosuppression necessary?
Immunosuppression therapy is standard for non-neural allograft operations including kidney, pancreas, heart, and liver transplantation. However, experience with human to human transplantation of either brain or retinal cells, suggests that immunosuppressant drugs are not required for graft survival in these relatively immunologically privileged sites [33-36]. Accordingly, in this initial human series we decided to avoid use of systemic immunosuppressive agents and systemic corticosteroids. Topical application of corticosteroids was limited to the immediate post-operative period. It remains for future studies to indicate whether immunosuppression, whether sustained or temporary, will increase the success rate of retinal transplantation. Evidence gathered FROM those studies will help to determine when and if, the danger of side effects outweigh the benefits of such therapy.
Is it ethical to use fetal issue tissue for transplantation?
We perceive no ethical dilemma in the proper use of fetal tissue. Abortion is legal in India and in the USA. The women who donate the tissue have made the decision to terminate their pregnancies that is totally independent of the transplantation procedure. That decision was made in consultation with their physicians following approved rules and regulations. The donors approved the use of the tissue for transplantation and the patients signed consents for being grafted with fetal tissue. There wa no compensation whatsoever to the donor or to the obstetrician. All the fetal tissues not used for transplantation, are simply discarded as medical waste. In fact, all of the tissue, except for the small amount we transplant is discarded. The protocols used in our studies have been approved by the review boards of the Wilmer Institute, the Prasad Institute and the University of Rochester. Nonetheless, we are looking for alternatives, and we have found it in the case of RPE cells used for experimental studies [37].
Are there alternatives to fetal neural tissue for transplantation?
Currently, other groups are using adult neural retinal cells for transplantation. However, we have had little success in adult neural tissue surviving transplantation. Also, there is very limited laboratory DATA on the survivability of adult neurons post-transplantation. By far, the most successful neural transplantation work has employed fetal tissue [1,2,21,36,38].
We have investigated in the laboratory the possible use of cell lines. One very well studied cell line is the Y79 line which was derived FROM retinoblastoma cells. We have shown that it is possible to modify these cells and use them with excellent results in experimental neural retinal transplantation [39]. Of course, the use of tumor-derived cells for human application is out of question. In sum, at this point laboratory evidence indicates that it may be possible and effective to transplant human patients with adult donor RPE cells, but the finding of suitable alternatives for photoreceptor transplantation remains in the future. This is one more example of the need for continued laboratory research to support and improve human transplantation.
Is transplantation to be used to REPLACE already lost retinal cells or to rescue cells before they die?
The answer based on existing experimental DATA is that both purposes can be served by transplantation. Of course, different conditions, and even different stages of the same condition will require different strategies. In the case of neural retinal transplantation, the cells are used to REPLACE lost photoreceptor cells. In the case or RPE transplantation, the cells used for the anatomical or functional replacement of lost or damaged RPE cells. This replacement may in turn rescue remaining photoreceptor cells. In both cases, a functioning inner retina is a prerequisite.
Diseases such as retinitis pigmentosa and AMD are prime candidates for transplantation treatment. The requirements for successful neural transplantation in RP are dependent on the existence of a functioning inner retina and to the presence of some remaining plasticity in the host retina. It is to be noted in this regard, that electrical stimulation of the retina in RP patients has shown that indeed the inner retina circuitry is preserved and that an accurate description of the stimulus shape is possible [29]. Plasticity has been demonstrated to remain present even in advanced states of the disease [27,30] as discussed above.
In AMD, the situation is more complex and there are at least four therapeutic requirements to be met. These include the REPAIR of Bruchís membrane, the replacement of dead or sick RPE cells, and the repopulation of photoreceptors. In addition, it will be necessary to prevent the degeneration FROM recurring within a reasonable time frame. Accomplishment of these various goals will demand a deeper knowledge of the pathophysiology of the disease.
The Wilmer study and the Hyderabad study. Similarities and differences.
Tables 2 and 3 summarize the similarities and differences between the two studies.
Regarding the differences, the patients were different both in age. Although not proven, it is likely that the American and Indian patients carry different mutations. The patients were operated by two different surgical teams. Slight differences in surgical technique may account for small differences in the outcome. One of those differences is the use of endophotocoagulation in only one of the surgical centers. The tissue was stored for different lengths of time in India and in the USA. We know that the time of storage does not significantly affect the viability of the cells, but does it affect their ability to differentiate as photoreceptors after transplantation? The answer to this question requires further laboratory research. We plan to experiment with that variable. Finally, we are still dealing with too small of numbers in each series to draw statistically valid conclusions about differences between the two groups.
Future directions in neural retinal transplantation.
A number of parameters are likely to be changed in searching for efficacious human retinal transplants. Some are obvious at this time, others will present themselves as research progresses. Certainly, one possible line of research is to search for a dose-effect response curve by increasing the number of cells transplanted at a given site. Also, the functional correlates of multiple graft sites within one eye need to be determined. Whether an increase in the number of grafted cells will result in a more pronounced functional response will need to be determined in animals and eventually in human patients. The possible immunological response to an increased allogenic LOAD needs will need to be addressed.
The eye has long been know to be an immune privileged site and recently the rejection of tissue within the ocular environment has been shown to occur through different mechanisms than those operating in the rest of the body. A non-inflammatory, gradual degeneration of the grafted tissue has been shown which could be due to non-classical immune mechanisms or other causes such as lack of appropriate trophic factors. However, until autopsy or biopsy tissue is available the answer will not be known.
Neural retinal cells transplanted thus far have not been pre-treated in any specific manner and neither have the hosts. We have recently started a limited, phase 1 study for testing the effects of a calcium channel blocker on human neural retinal transplants [40]. This work is predicated on the research with nimodipine in neural transplantation for Parkinsonís Disease. This has shown that such treatment may be of some benefit to the grafted tissues with little risk and no harm to the patient [41,42]. Although no deleterious effect was observed so far in our patients, substantially longer follow ups will be required before any firm conclusion may be derived FROM this attempt.
Any new experimental or surgical approach will need to rest on objective, quantitative evaluations of the patientsí vision. A newly designed test battery has recently been described to maximize detection of any changes that occur in transplanted patients with extremely low vision [7]. Such tests have already being implemented at both Wilmer and Prasad. Consistent implementation of these tests will permit the biostatistical analysis required for human retinal transplantation to progress to a second, more advanced phase. | | | Acknowledgements | The work presented in this synopsis is based on the crucial collaboration with the surgical teams FROM the Wilmer Eye Institute (Eugene de Juan, Jr., M.D., Mark Humayun, M.D., Gislin Dagnelie, Ph.D.) and the L.V. Prasad Eye Institute (Taraprasad Das, M.D., Subhdra Jalali, M.D., Savitri Sharma, M.D.). Separate manuscripts detailing the results FROM each institution are in preparation.
This work was supported by private gifts to the University of Rochester and the Wilmer Eye Institute, the Hyderabad Eye Research Foundation, the Grousbeck Foundation, the Rochester Eye and Human Parts Bank, and Research to Prevent Blindness. | | | References | 1. del Cerro, M., Retinal transplants. In: Progress in Retinal Research, Osborne, N., Chader, G., eds., Pergamon Press: New York (1990).
2. del Cerro, M., Lazar, E.S., DiLoreto, D., The first decade of continuous progress in retinal transplantation. Microscopy Research and Technique 36: 130-141 (1997).
3. de Juan E, del Cerro M, Dagnelie G, Humayun, M., del Cerro, C., DiLoreto, D., Greenberg, R., Neural retinal transplantation: a phase I clinical trial. Invest Ophthalmol Vis Sci 38: S261 (1997).
4. del Cerro, M., Das, T., Lazar, E., Jalali, S., DiLoreto, D., Sreedharan, A., Rao, V.S., Little, C., Sharma, S., del Cerro, C., Rao, G.N., Human fetal neural retinal cell transplantation in retinitis pigmentosa. Vision Res 35: S140 (1995).
5. Das, T., del Cerro, M., Lazar, E., Jalali, S., DiLoreto, D., Sreedharan, A., Rao, V.S., Little, C., Sharma, S., del Cerro, C., Rao, G.N, Transplantation of neural retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 27: S96 (1996).
6. DiLoreto, D., del Cerro, C., Lazar, E., Cox, C., del Cerro, M., Storage of human fetal retina in Optisol prior to subretinal transplantation. Cell Transplant 5: 553-561 (1996).
7. Dagnelie, G., Sunness, J.S., de Juan, E., Humayun, M., A test battery to monitor visual function in blind volunteers for retinal cell transplantation. Invest Ophthalmol Vis Sci 38: S333 (1997).
8. Marmor, M.F., Arden, G.B., Nilsson, S.E., Zrenner, E., Standard for electroretinography. Arch Ophthalmol 107: 816-819 (1989).
9. Allergan Humphrey, The Field Analyzer Primer, Haley, M., ed. (1987).
10. Bird, A.C., Inherited outer retinal dystrophies. In: Tasman, W.S., ed., Clinical Decisions in Medical Retinal Disease, Mosby: St.Louis (1994).
11. Berson, E.L., Rosner, B., Sandberg, M.A., Hayes, K.C., Nicholson, B.W., Weigel-Difranco, C., Willet, W., A randomised trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 111: 761-772 (1993).
12. Marmor, M.F., A randomised trial of vitamin A and vitamin E supplementation for retinitis pigmentosa (correspondence). Arch Ophthalmol 111: 1460-1461 (1993).
13. Norton, E.W.D., A randomised trial of Vitamin A and Vitamin E supplementation for retinitis pigmentosa. (correspondence). Arch Ophthalmol 111: 1460 (1993).
14. Kolb, H., Gouras, P., Electron microscopic observations of human retinitis pigmentosa, dominantly inherited. Invest Ophthalmol Vis Sci 13: 487-498 (1974).
15. Szamier, R.B., Berson, E.L., Retinal ultrastructure in advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci 16: 947-962 (1977).
16. Szamier, R.B., Berson, E.L., Klein, R., Meyers, S., Sex linked retinitis pigmentosa: ultrastructure of photoreceptors and pigment epithelium. Invest Ophthalmol Vis Sci 18:145-160 (1979).
17. Gartner, S., Henkind, P., Pathology of retinitis pigmentosa. Ophthalmol 89: 1425-1432 (1982).
18. Meyer, K.T., Heckenlively, J.R., Spitznas, M., Foos, R.Y., Dominant retinitis pigmentosa. A clinicopathological correlation. Ophthalmol 89: 1414-1424 (1982).
19. Green, W.R., Retina. In: Ophthalmic Pathology Vol. III, Spencer, W.H., ed., WB Saunders: Philadelphia (1985).
20. del Cerro, M., Notter, M.F.D., del Cerro, C., Weigand, S.J., Grover, D.A., Lazar, E., Intraretinal transplantation for rod-cell replacement in light-damaged retina. J Neural Transplant 1: 1-10 (1989).
21. Gouras, P., Du, H., Gelanze, M., Lopez, R., Kwun, R., Kjeldbye, H., Krebs, W., Survival and synapse formation of transplanted rat rods. J Neural Transplant 2: 91-100 (1991).
22. Gouras, P., Du, H., Kjeldbye, H., Zack, D.J., Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest Ophthalmol Vis Sci 33: 2579-2586 (1992).
23. del Cerro, M., Ison, J.R., Bowen, G.P., Lazar, E., del Cerro, C. Intraretinal grafting restores visual function in light-blinded rats. Neuroreports 2: 529-532 (1991).
24. Ho, T.C., Del Priore, L.V., Reattachment of cultured human retinal pigment epithelium to extracellular matrix and human Bruchís membrane. Invest Ophthalmol Vis Sci 38: 1110-1118 (1997).
25. Fariss, R.N., Li, Z.Y., Milam, A.H., Evidence for plasticity in human RP patients. Invest Ophthalmol Vis Sci 38: S260 (1997).
26. Santos, A., Humayun, M.S., de Juan, E., Greenberg, R.J., Marsh, M.J., Klock, I.B., Milam, A.H., Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Invest Ophthalmol Vis Sci 38: S309 (1997).
27. Peng, M., Wen, R., Increase in bFGF and CNTF in rat retina induced by mechanical injury. Invest Ophthalmol Vis Sci 38: S604 (1997).
28. Cao, W., Li, F., LaVail, M.M., Steinberg, R.H., Development of injury-induced gene expression of bFGF, FGR-1, CNTF, and GFAP in rat retina. Invest Ophthalmol Vis Sci 38: S604 (1997).
29. Humayun, M., de Juan, E., Dagnelie, G., Greenberg, R.J., Propst, R.H., Phillips, D.H., Visual perception elicited by electrical stimulation of the retina in blind humans. Arch Ophthalmol 114: 40-46 (1996).
30. Li, Z.Y., Kljavin, I.J., Milan, A.H., Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci 15: 5429-5438 (1995).
31. Facktorovich, E.G., Steinberg, R.H., Yasumura, D., Matthes, M.T., LaVail, M.M., Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347: 83-86 (1990).
32. Hicks, D., Courtois, Y., Fibrolast growth factor stimulates photoreceptor differentiation in vitro. J Neurosci 12: 2022-2033 (1992).
33. Algvere, P.V., Berglin, L., Gouras, P., Sheng, Y., Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefeís Arch Clin Exp Ophthalmol 232: 707-716 (1994).
34. Henderson, B.T.H., Clough, C.G., Hughes, R.C., et al., Implantation of human fetal ventral mesencephalon to the right caudate nucleus in advanced Parkinsonís disease. Arch Neurol 48: 822-827 (1991).
35. Sawle, G.V., Bloomfield, P.M., Bjorklund, A., et al., Transplantation of fetal dopamine neurons in Parkinsonís disease: PET [18F]6-L-fluorodopa studies in two patients with putarninal implants. Ann Neurol 31: 166-173 (1992).
36. Freed, C.R., Breeze, R.E., Rosenberg, N.L., Schneck, S.A., Kriek, E., Qi, J.X., Lone, T., Zhang, Y.B., Snyder, J.A., Wells, T.H., Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinsonís disease. N Eng J Med 327: 1549-1555 (1992).
37. Castillo, B.V., del Cerro, M., White, R.M., Cox, C., Wyatt, J., Nadiga, G., del Cerro, C., Efficacy of non-fetal human RPE for photoreceptor rescue: a study in dystrophic RCS rats. Exp Neurol in press (1997).
38. Kordower, J.H., Freeman, T.B., Snow, B.J., Vingerhoets, F.J., Mufson, E.J., Sanberg, P.R., Hauser, R.A., Smith, D.A., Nauert, G.M., Perl, D.P., Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. New Eng J Med 332: 1118-24 (1995).
39. del Cerro, M., Seigel, G.M., Lazar, E., Grover, D., del Cerro, C., Brooks, D.H., DiLoreto, D., Chader, G.J., Transplantation of Y79 cells INTO rat eyes: an in vivo model of human retinoblastomas. Invest Ophthalmol Vis Sci 34: 3336-3346 (1993).
40. del Cerro, M., Das, T.P., Lazar, E.S., del Cerro, C., Sreedharan, A., Sharma, S., Rao, G.N., Neural retinal transplantation INTO twelve RP patients. Innvest Ophthalmol Vis Sci 38: S261 (1997).
41. Finger, S., Dunnet, S., Nimodipine enhances growth and visualization of neural grafts. Exp Neurol 104: 1-9 (1989).
42. Zhou, F.C., Pu, C.F., Finger, S., Nimodipine-enhanced survival of suboptimal neural grafts. Restor Neurol Neurosci 3: 211-15 (1991). | | | Tables | Table 1 summarizes the pre- and post-operative visual acuity of the eye that was transplanted.
Table 1: Prasad study: patient characteristics of operated eye
Patient |
Age |
Sex |
Pre-op VA |
Follow-up |
Post-op VA |
Post-op VF |
|---|
1 |
26 |
M |
LP |
29 m |
HM |
--- |
2 |
24 |
M |
LP |
29 m |
LP |
--- |
3 |
32 |
M |
LP |
29 m |
20/200 |
3 |
4 |
35 |
M |
LP |
23 m |
LP |
--- |
5 |
35 |
F |
LP |
23 m |
HM |
--- |
6 |
32 |
F |
LP |
17 m |
CF, 15 cm |
--- |
7 |
35 |
F |
LP |
17 m |
LP |
--- |
8 |
40 |
M |
CF, 15cm |
17 m |
CF, 50cm |
1.5 |
9 |
40 |
M |
HM |
10 m |
LP |
--- |
10 |
25 |
M |
LP |
10 m |
LP |
--- |
11 |
35 |
M |
LP |
10 m |
HM |
--- |
12 |
30 |
M |
LP |
10 m |
LP |
--- |
"2">
Table 2: Interstudy Similarities
|
Parameter |
Wilmer & Prasad |
|---|
Donor age (gestational weeks) |
14 to 18 |
Tissue storage medium |
Optisol (Chiron) |
Tissue dissociation |
Mechanical to single cells and small clusters |
Vehicle |
DMEM (Gibco) |
Delivery device |
Two function manipulator (Grieshaber) |
Transplant site |
Parafoveal |
Systemic Immunosuppression |
None |
"2">
Table 3: Interstudy Differences
|
Parameter |
Wilmer |
Prasad |
|---|
Age (yrs ±SD) |
54 ± 15 |
32 ± 5 |
Tissue storage (hours) |
< 72 |
< 24 |
Electrical stimulation |
Yes |
No |
Photocoagulation |
No |
Yes |
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in