|
|
 |
 |
 |
 |
|
|
RPE and Photoreceptor Transplantation
Digital Journal of Ophthalmology 1998
Volume 4, Number 6
September 30, 1998
|
Printer Friendly
|



Peter Gouras, M.D. | Harkness Eye Institute, Columbia University Peep V. Algvere, M.D. | St. Erik's Hospital, The Karolinska Institute
|
|
|
| Abstract | Objective
This paper reviews the status of RPE (retinal pigment epithelium) and photoreceptor transplantation. RPE transplants succeed in rescuing photoreceptors by replacing defective RPE in the RCS rat. So far this technique has not been successful in treating age-related macular degeneration (AMD). This may be due to the fact that RPE is slowly rejected in such patients. Immunosuppression therapy is being used to test this possibility.
Methods
There is no animal model demonstrating the effectiveness of photoreceptor transplantation. We have been testing this approach in the rd mouse. Although neonatal murine photoreceptors survive transplantation for long periods, synaptic communication with host neurons is impeded by Muller cell processes blocking access to the inner nuclear layer. Strategies are needed to overcome this barrier.
So far two approaches that have been used to promote the idea that transplanted retinal cells can REPLACE dying or defective host cells. One involves the transplantation of retinal epithelium; the other photoreceptors.Keywords
retinal pigment epithelium, rats transplantation | | | Discussion | Retinal pigment epithelium (RPE) transplantation
Retinal pigment epithelium (RPE) transplantation is conceptually simpler because the RPE is only a monolayer of cells between the systemic circulation and the photoreceptors. It works by being next to the outer segments of the photoreceptors, separated by a narrow extracellular space, uniquely enriched with specialized proteins. Although all of the functional roles of the RPE have not yet been elucidated, most of the major ones have. We know that without an RPE layer, the photoreceptors as well as the choriocapillaris degenerate. The utility of RPE transplantation has been demonstrated by the RCS rat model. Here defective RPE leads to photoreceptor degeneration. Transplanted RPE corrects this defect and prevents the photoreceptor cells FROM degenerating. The therapeutic effect is long-lived and there is only mild evidence of rejection (Bok et al, personal communication). Transplantation of RPE works even though the transplanted cells are merely layered over the defective host RPE. There is no need to remove the defective cells and the multilayering that occurs as a result of such transplantation does not appear detrimental to the host photoreceptors.
It was at the previous Fernstrom symposium on the retina a decade ago that we reported that transplanted RPE was able to phagocytize outer segments in a foreign host retina and stated that a possible treatment of the RCS rat model retinal degeneration was testable [1]. Within two years both our laboratory and Jim Turner's had succeeded in demonstrating that this worked [2-4]. It remains so far the only successful demonstration of the potential efficacy of retinal cell transplantation. It was this success in the RCS rat that suggested the possibility that some forms of human retinal degeneration, presumably due to defective RPE, might also be treatable by RPE transplantation. This has led to a collaborative study with Peep Algvere at the Karolinska Hospital in Stockholm in attempts to test this hypothesis in patients with age-related macular degeneration (AMD) [5-6].
These human experiments are being impeded by what we believe to be host/graft rejection. This occurred in the first GROUP of patients within 1-2 months after transplantation. These patients had submacular neovascular tissue removed just before "patch" transplants of cultured fetal human RPE were inserted. Such transplants were essentially placed on the vascular choroid and therefore deprived of any immunological privilege. A second GROUP of patients with advanced "dry" forms of AMD, and therefore without any neovascular membranes, received relatively small (0.6 mm diameter) transplants placed paramacularly. These transplants have not rejected now two years after surgery. In ORDER to try to cover larger areas, however, and include the fovea, we resorted to concentrated fetal human RPE cell suspensions. This approach, however, has also led to what appears to be rejection, but over a slightly slower time course.
In ORDER to explore the tolerance of the subretinal space in primates to RPE transplantation, we transplanted human fetal RPE to the paramacula and foveal areas of monkeys. We found that most of these paramacular RPE xenografts survived for at least 6 months but most of the foveal ones rejected [7]. We speculated on several possible causes for the greater frequency of rejection of foveal versus non-foveal RPE transplants. It is more difficult to produce a bleb detachment in the monkey fovea, which could lead to greater surgical trauma, inflammation and consequently rejection. The choroidal blood supply is also more extensive than it is in more peripheral regions of the retina. Both of these factors could contribute to the greater rejection vulnerability of foveally placed transplants.
The recent studies of foveally placed RPE cell suspension transplants in patients with advanced AMD suggest that it is not the surgery but the vulnerability of the fovea to rejection because it is easy to produce a bleb detachment in such patients and we know in addition that perimacular transplants in bleb detachments that include the fovea are not rejected for at least two years after transplantation. Therefore, we favor the hypothesis that the increased choroidal circulation may be responsible for the greater incidence of RPE allograft rejection at the fovea.
We have only transplanted fetal and usually cultured human RPE. It seems unlikely that fetal would be more immunoprovocative than fresh or cultured adult RPE [8]. Therefore in ORDER to transplant healthy RPE to the foveal area of patients with relatively advanced AMD, it is necessary to stop rejection. We are now using standard immunosuppression in our attempts to treat AMD with RPE transplants. It is conceivable that immunosuppression may only be needed for a short time during and after transplantation surgery but this can only be answered by further trials. We have also found that local injection of cyclosporine can prevent RPE xenograft rejection in rabbits [9]. There are other ways to eliminate rejection, such as the use of homotransplants of RPE or iris pigment epithelium. Another possibility that should develop with further progress in genetic engineering is to either knockout the genes expressing surface antigens on RPE cells or coat the transplant cells with antibodies that block their detection. At earlier stages of AMD where the blood/brain barrier may not be disturbed, host/graft rejection may be less problematic.
Photoreceptor Transplants
Here we have no animal model that demonstrates its effectiveness. We have been using the rd mouse, which is a rapid photoreceptor degeneration due to a gene defect in a rod specific enzyme. We have found the best way to establish healthy appearing photoreceptor transplants in the subretinal space of this mice is to transplant 1-2 day neonatal progenitor photoreceptor cells as small microaggregates [10]. Most of our work has involved microaggregates that also include the inner retinal layers of the retina because our main goal had been to determine the long term survival of photoreceptor transplants. Such progenitor photoreceptors, both rod and cones, will develop normally and survive indefinitely in the host rd retina; our longest survival time is 9 months [10]. It is essential, however, to have the progenitor photoreceptor cells properly oriented toward the host's RPE layer. If they are oriented toward the inner retinal layers of the host, the photoreceptors degenerate and are phagocytized by macrophages [10]. If the microaggregates are too large they tend to form rosettes and again, the photoreceptors degenerate. Therefore success in photoreceptor transplantation requires that the developing photoreceptor be appropriately oriented to the RPE layer. This had not yet been shown by other research in this field [11-14]. Approaches using, for example, gelatin slabs to guarantee photoreceptor polarity are therefore good ideas [15].
The major question we have been addressing now is the communication of such transplants with the inner retinal neurons of the host. We have been examining this anatomically because it provides a two- and three- dimensional view of the interface between transplant and host neurons. Neurophysiology provides a functional but essentially one- dimensional view of the interacting systems. In ORDER to facilitate identification of what are transplanted rods and what are host rod bipolars we have been working with two transgenic mouse strains. The donors, provided by Donald Zack, express the lac Z reporter gene strongly in their rods. The host mice, provided by Jim Morgan, express the same reporter gene in their rod bipolars. We have bred the latter mice INTO the rd/rd strain, producing virtually photoreceptorless mice with lac Z labeled rod bipolars.
Because we are attempting to facilitate transplanted photoreceptor to host bipolar synaptic contacts we cut away most or all of the inner retinal layers of the donor tissue before transplantation with a vibratome.
First of all there is no host/graft rejection. We find relatively close contact between the transplanted photoreceptors and the inner nuclear layer neurons of the host but there is a barrier to intimate contact due to Muller cell growth subsequent to the degeneration of the host photoreceptor layer. This barrier is formed by junctional complexes between the terminals of Muller cells including a profuse network of basket fibers produced by the ends of these terminal processes (Fig. 1).
There are occasionally areas where a labeled transplanted rod perikaryon intrudes across this barrier INTO the host territory (Fig. 2). Therefore the barrier is penetrable.
Occasionally rod axons also appear to be crossing this barrier (Fig. 3). This is concluded because these terminals contain X-gal particles that label the donor rods (Fig. 3, arrowhead).In examining the post-synaptic structures these labeled processes are contacting, it is not often clear to what cells they belong. The reason for this is that the post synaptic structures are small and most do not contain lac Z particles which should exclusively label host rod bipolars. In rare cases lac Z particles are found in a post synaptic structure contacting a labelled donor rod spherule (Fig. 4).
In this particular case, the synapse is located in the transplant territory, implying that the post synaptic process, i.e. FROM a presumed host rod bipolar, has grown FROM the host INTO the transplant. The particles produced by X-gal, however, often straddle the pre- and post-synaptic membrane making it uncertain that they really belong to the small post synaptic structure or are really pre-synaptic. In ORDER to answer this important question unambiguously, serial sectioning will be required at the EM level to determine the perikaryon of these post synaptic processes. Are they unequivocally host rod bipolars or other host neurons or are they residual inner nuclear cells of the donor?
What we can unequivocally conclude is that photoreceptor transplants survive quite well when they are placed in the proper position relative to the RPE. This in itself is good news. Synaptic communication, however, between the donor photoreceptors and the host bipolars is greatly handicapped due to a Muller cell gliosis. How this barrier can be overcome will require a lot of imaginative research in the future but the stakes are high since any success offers the possibility of restoring vision to a blind eye.
One other consideration is also worth noting in photoreceptor transplantation experiments. The, as yet unexplained reason, why cones die in an hereditary gene defect that is exclusive to rods, as in the rd mouse, suggests that if these cones were next to, near or within diffusing distance FROM normal rods, their rate of degeneration could be slowed or even stopped. We have been examining this in our photoreceptor transplant experiments. We are evaluating the ultrastructure of residual cones in similar areas of the contralateral control retina and comparing them to host cones in and around the transplant area. This research is too preliminary to make significant conclusions. What we have found so far in a 2-3 month old rd control retina are cones with slightly to severely degenerate nuclei, with underdeveloped cone pedicles, relatively healthy inner segments (Fig. 5), a cilium and occasionally minute stack of outer segment disks (Fig. 6) confirming earlier research [15,16].
The appearance and number of such cones appears qualitatively similar in and around retinal areas that have received photoreceptor transplants. Continued and probably even more sophisticated examinations may be required to test whether healthy photoreceptor transplants can trophically alter secondary cone degeneration in the rd mouse. | |

Figure 1
An X-gal labeled donor rod transplant (T) in the subretinal space of a 2 1/2 month old rd mutant mouse that received this transplant at 24 days after birth. X-gal labeled rod bipolars (H) can be seen on the opposite side of the external limiting membrane (ELM) formed by junctional complexes (arrow) and basket-like processes (*) of Muller cells.
|
|
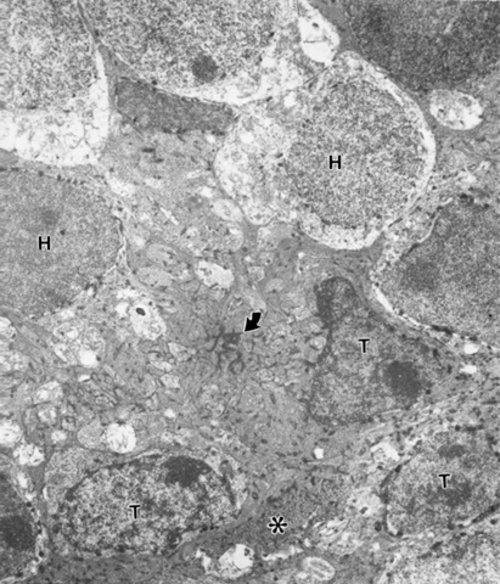
Figure 2
An X-gal labeled donor rod perikaryon (T) penetrating the ELM (arrow) with basket-like processes (*) of Muller cells. Other transplanted rod (Ts, below) and X-gal labeled host rod bipolars (H). FROM same mouse of Figure 1.
|
|
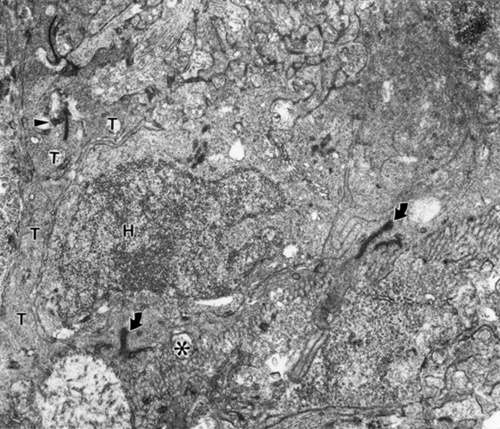
Figure 3
An X-gal labeled (arrowhead) donor rod axon terminal penetrating INTO host retina, demarcated by junctional complexes (arrows) and basket processes (*) of ELM. The host retina (H) is above and the transplant below the ELM. Same mouse as in Figure 1.
|
|

Figure 4
A synaptic terminal (T) of an X-gal labeled (arrowhead) donor rod terminal with an X-gal labeled (arrowhead) particle in a postsynaptic structure. A labeled rod perikaryon (T) is below and the host (H) retina above the ELM with its junctional complexes (arrow) and basket processes (*). Same mouse as in Figure 1.
|
|
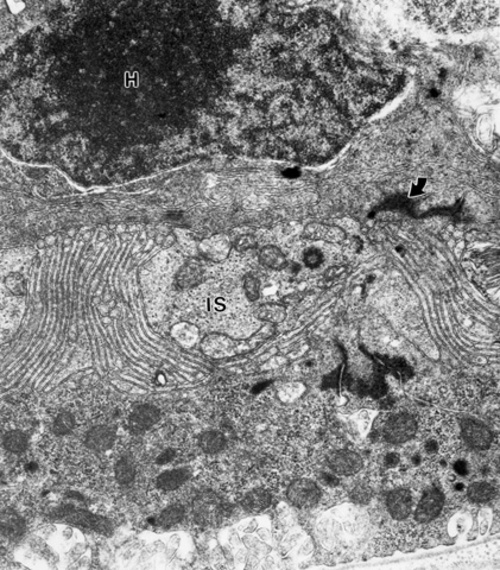
Figure 5
The contralateral control eye of the mouse of Figures 1-4 showing a cone perikaryon (H) and an inner segment (IS) below the ELM (arrow).
|
|

Figure 6
The control eye of the mouse of Figs. 1-4 showing some outer segment material near a host cone (H) below the ELM with junctional complexes (arrows).
|
|
| References | 1. Gouras, P., Lopez, R., Brittis, M., Kjeldbye, H., Fasano, M.K., Transplantation of cultured retinal epithelium. in Retinal Signals Systems, Degenerations and Transplants. E. Agardh and B. Ehinger eds. pp 271-286. Elsevier (1986)
2. Reppucci, V., Goluboff, E., Wapner, F., Syniuta, L., Brittis, M., Sullivan, B., Gouras, P., Retinal pigment epithelium transplantation in the RCS rat. Investigative Ophthalmology & Visual Science Suppl 29:144 (1988)
3. Li, L., Turner, JE., Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Experimental Eye Research 47: 911-947 (1988)
4. Lopez, R., Gouras, P., Kjeldbye, H., Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Investigative Ophthalmology & Visual Science 30:586-588 (1989)
5. Algvere, PV., Berglin, L., Gouras, P., Sheng, Y., Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefe's Archiv Clinical Experimental Ophthalmology 232:707-716 (1994)
6. Algvere, PV., Berglin, L., Gouras, P., Sheng, Y., Human fetal RPE transplants in age-related macular degeneration (ARMD). Graefe's Archiv Clinical Experimental Ophthalmology 235:149-158 (1997)
7. Berglin, L., Gouras, P., Sheng, Y., Lavid, J., Lin, PK., Cao, H., Kjeldbye, H., Tolerance of human fetal RPE xenografts in monkey retina. Graefe's Archiv Clinical Experimental Ophthalmology 235:103-110 (1997)
8. Dafgard-Kopp, EME., Winter-Vernersson, AM., Algvere, PV., Expression of HLA class I and class II antigens in human fetal RPE cells. Investigative Ophthalmology & Visual Science 38(4):5947 (1997)
9. Sheng, Y., Li, W., Cao, H., Lin, PK., Lavid, J., Saeki, M., Gouras, P., Intravitreal cyclosporine prevents RPE xenograft rejection in rabbit retina. Investigative Ophthalmology & Visual Science 36(4):S250 (1990)
10. Gouras, P., Du, J., Kjeldbye, H., Yamamoto, S., Zack, DJ., Long-term photoreceptor transplants in dystrophic and normal mouse retina. Investigative Ophthalmology & Visual Science 35:3145-3158 (1994)
11. Aramant, R., Seiler, M., Ehinger, B., Bergstrom, A., Gustavii, B., Brundin, P., Adolph, AR., Transplantation of human embryonic retina to adult rat retina. Rest Neurology and Neuroscience 2:9-22 (1990)
12. Ehinger, B., Bergstrom, A., Seiler, M., Aramant, RB., Zucker, C., Gustavii, B and Adolph, AR., Ulstrastructure of human retinal cell transplants with long survival times in rats. Experimental Eye Research 53:447-460 (1989)
13. delCerro, M., Noter, M., delCerro, C., Wiegand, S., Grover, D., Lazar, EJ., Intraretinal transplantation for rod-cell replacement in light-damaged retinas. Journal of Neural Transplantation 1:19 (1989)
14. Silverman, MS., Hughes, SE., Transplantation of photoreceptors to light-damaged retina. Investigative Ophthalmology & Visual Science 30:1684-1690 (1989)
15. Sanyal, S., Bal, AK., Comparative light and electron microscopic study of retinal histogenesis in normal and rd mutant mice. Z. Anat. Entwickl. Gesch 142:219-238 (1973)
16. Carter-Dawson, LD., LaVail, MM., Sidman, RL., Differential effect of the rd mutation on rods and cones in the mouse retina. Investigative Ophthalmology & Visual Science 17:489-498 (1978) | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in