|
|
 |
 |
 |
 |
|
|
Humoral Response In Herpes Keratitis Patients : Results Of A South Indian Study
Digital Journal of Ophthalmology 1998
Volume 4, Number 9
October 30, 1998
|
Printer Friendly
|


 Naranatt Padmanabhan Pramod
Naranatt Padmanabhan Pramod | Dr. ALM Post Graduate Institute of Basic Medical Sciences Sadras Panchachiram Thyagarajan | Dr. ALM Post Graduate Institute of Basic Medical Sciences Ananda Kannan | Regional Institute of Ophthalmology
|
|
|
| Abstract | Objective
Serum IgG against Herpes simplex virus(HSV) were quantitated in 234 clinically suspected herpes simplex keratitis(HSK) patients. A paired serum sample was available only in 56 cases. Over a 3 week interval non-diagnostic fluctuations of HSV IgG occurred in many patients. The utility of a single serum sample was also looked at by analyzing sera of 60 voluntary blood donors. This study shows the status of usefulness of serum IgG against HSV in herpes keratitis.Keywords
Herpes simplex virus, keratitis, humoral response | | | Introduction | | Herpes simplex virus(HSV) is an important ocular pathogen and is acknowledged as a major cause of corneal blindness in developed countries(1,2). HSV can affect the cornea in a number of ways, commonly producing the self limiting epithelial disease in the form of dendritic ulcer which can lead to prolonged inflammatory disease in the corneal stroma, with eventual development of scarring in a significant proportion of the patients(3). Serological response to primary ocular HSV infection involves the production of HSV IgM antibodies followed by IgG and IgA antibodies(4). Subsequently HSV IgG may persist for a long period and this marker may be useful in estimating the populations exposure to HSV. Most of the ocular infections are believed to be recurrent HSV infection per se(5) as most of the patients have had some previous contact with HSV and hence circulating antibodies. But despite circulating antibodies people may later develop recurrent keratitis(6). We wanted to determine if HSK is associated with any anamnestic humoral immune response. We report the results of our investigation INTO humoral response in patients with epithelial and stromal keratitis, who are compared to a control group. | | | Materials and Methods | 1. Patients and materials:
234 patients with clinically suspected HSK referred to the cornea service of Regional Institute of Ophthalmology, Madras ,India during the period 1994-97 were studied. The clinical material consisted of corneal scrapings and serum samples. A paired serum sample could be collected only in 56 cases (at an interval of 3-4 weeks). The corneal scrapings were collected with sterile Bard-Parker blade No.15 and transported in Hanks Balanced Salt Solution (HiMedia, Bombay, India) with antibiotics and 3% Fetal Bovine serum (Sigma Chemical Co., St.Louis, MO, USA). All specimens were transported on ice and processed immediately and wherever there was a delay they were stored in - 70 deg C until processed. The laboratory investigations included HSV isolation, detection of HSV specific antigen and quantitation of anti-HSV IgG in all the single and paired serum samples.
2. Virus isolation:
Isolation of HSV was done as per the procedure described by Kaye et al (7) using Vero cell line (National Centre for Cell Science, Pune, India). A positive and a negative control virus, a standard strain (strain No. 753166, National Institute of Virology, Pune, India) of HSV-1 and viral transport medium respectively were included with every batch of the test. The cultures were observed daily for the development of characteristic Cytopathic effect (CPE) of HSV (8) under an inverted phase contrast microscope (Nikon Diaphot, Nikon Corporation, Tokyo, Japan). Smears were made FROM all test tubes showing CPE and virus was identified by direct immunofluorescence (Syva Microtrack kit) test. All negative cultures were passaged once at the end of 7th day before declaring the specimen negative.
3. Antigen detection:
Corneal scrapings were subjected to an in-house, indirect IF test for HSV antigens as per the method of by Boerman et al (9) with modifications. About 1ml of the specimen in viral transport medium was centrifuged (10 min at 100 g) and the sediment was dissolved in 50 microl of Phosphate Buffered Saline (PBS, pH 7.2) and applied on a clean glass slide. Slides were fixed in cold acetone and fluorescent staining was performed. HSV-1 infected and uninfected Vero cells were used as controls. The primary and secondary antibody used were polyclonal Rabbit anti-HSV antibodies (strain McIntyre, Dako a/s, Denmark) and anti-Rabbit IgG FITC (National Institute of Immunology, New Delhi, India) respectively. The slides were then counterstained with 0.0001% Evans Blue (HiMedia), rinsed in distilled water and mounted in Buffered Glycerol saline. Optimum working dilutions of the reagents were determined by previously titrating each batch of reagents. The slides were examined under Nikon fluorescence microscope (Optiphot-2 with an EF-D epi-illuminator and a 100 W Hg lamp). The criterion of positivity was the detection of one or more cells with bright apple green fluorescence in cytoplasm or nucleus or both.
4. Anti-HSV IgG assay:
Serum samples were tested for anti-HSV IgG levels by an "in-house" ELISA developed as per Peter Fox et al (10)with modifications. Briefly, 96 well plates (Corning, USA)were coated with infected Vero cell lysate and control Vero antigen. Serum samples were diluted 1:2 in tris-HCl with 1% bovine serum albumin and incubated in antigen coated wells for 1hr at 37degree Celsius. Antigen specific IgG were detected by anti-human IgG HRP conjugate(National Institute of Immunology, New Delhi). After 1hr incubation enzyme substrate 3,3’, 5,5’-tetramethyl benzidine (Genei, Bangalore) was added and reaction was allowed to proceed for 30 minutes. The enzyme reaction was stopped by the addition of 2M H2SO4 and aborbance was measured at 450nm. Virus specific IgG concentration was calculated FROM a standard curve absorbance 450nm plotted against purified human IgG standard. This was constructed by incubation of anti-human IgG HRP conjugate in wells coated with dilutions of purified human IgG. After the initial runs the range was set at 2.5 microgram/ml to 160 microgram/ml. | | | Results | Clinical presentation:
Out of the 234 patients screened , 65 patients had laboratory evidence of HSV infection. The mean age was 29 years and the range was 9 months to 65 years. Male to female ratio was 2.1 to 1. The study population included 137 cases who presented with "first episode" of HSK and 97 had recurrent infection. The clinical characterization of these cases showed 153 had epithelial keratitis and 81, stromal infiltration. 92 cases were presented with dendritic ulcer (39.31%), 20 had geographic and 23, superficial punctate keratitis. Disciform and nectrotizing stromal keratitis was seen in 66 and 15 cases. 12 pseudodendritic and 6 cases with epithelial defect was also included (Table 1).
Thirty-eight "first episode" and 27 recurrent HSK had laboratory evidence of HSV infection. 50 had epithelial keratitis and 15 had stromal infiltration. Breakup of the clinical symptoms in laboratory proven cases showed 40 had dendritic ulcers. 2 had geographic ulcer and 4, superficial punctate keratitis. Marginal keratitis was seen only in 1 case. 12 and 3 of the patients presented with disciform and necrotizing stromal keratitis respectively.Atypical lesion and pseudodendritic lesion was seen in 2 cases each (Table 2). Ocular infection was unilateral in 64 cases and bilateral in only one case.
Tissue culture and antigen detection:
HSV was isolated FROM corneal scrapings of 36 cases. Of the 36 positive cases, 22 were "first episode disease" and 14 recurrent. Analyzing the virus isolation in terms of clinical presentation, the maximum isolation was seen in case of dendritic ulcer (24 nos.) followed by disciform keratitis (5 nos.) and geographic ulcer (2 nos.). One each was isolated FROM punctate and marginal keratitis. Two cases of pseudodendritic lesion and atypical clinical presentation also yielded HSV isolates. The number of days taken to develop CPE varied FROM 1 to 7 days. 16 (44.4%) of the HSV positive specimens gave a CPE by day 3 and in 55.55% of the cases CPE took as long as 4 to 7 days. An analysis of positive laboratory diagnosis with the duration between collection and onset revealed an indirect proportion in detection as the duration increased. In as many as 58% diagnosis was positive when the specimen was collected in £ 1 week which reduced to 5% as the time increased to 4 weeks. HSV specific antigen was detected by IF in 62 (26.49%) cases. The difference in sensitivity between cell culture and IF was found to be statistically significant (Mc Nemar’s test, P<0.05) (Table 3).
Serum IgG:
HSV specific IgG was measured by ELISA FROM the sera of 234 herpetic keratitis patients and 60 controls. The results are displayed in Fig 1.0, as analyzed in clinical groups. High levels of HSV IgG was detected in all the cases and controls. The results were analyzed in two groups. In the first GROUP HSV IgG was quantitated in all the single serum samples and a GROUP of 60 voluntary blood donors. The mean level of IgG being 17.37 +/-; 3.35 microgr/ml in the former and 16.31 +/-;3.7 microg/ml in the voluntary blood donor GROUP (Fig 1).
In the II Group, the paired sera analysis was taken-up. In the 56 cases were paired sera was available for analysis a raise in IgG quantity was seen in a total of 17 cases. This include 10 cases where HSV was laboratory proven and 7 cases where no laboratory evidence of HSV infection could not be found. The mean elevation was 4.0 microg/ml in the proven category and 2.07 microg/ml in the negative cases. The difference in the mean raise was however not statistically significant(Student’s’ test, p value, 0.07). There are reports of significantly high amounts of IgG in the dendritic GROUP when compared with other clinical groups and controls. However, such an analysis in our patients showed no significant difference among any of the clinical groups in epithelial keratitis or between epithelial or stromal keratitis. The mean antibody levels in the different clinical groups being 17.08 microg/ml in dendritic keratitis, 17.52 microg/ml in geographic, 26.62 microg/ml in superficial punctate keratitis and 18.48 microg/ml in stromal cases(Fig 2). Thus the mean titre was high in SPK when compared to other epithelial keratitis cases. However, the number of cases in SPK was small (ie. 23) when compared to other groups. | |
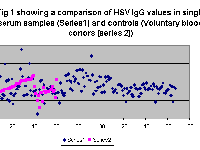
Figure 1
|
|
| Discussion | The objective of this study was to investigate whether the herpes epithelial or stromal disease is associated with any anamnestic humoral response. In the present series, high levels of HSV IgG was detected in all the various clinical groups of epithelial keratitis, stromal keratitis and in voluntary blood donor controls. In the paired sera analysis, out of the 56 cases analyzed, an elevation of IgG was seen in 17 pairs. However, the break-down of these cases showed 10 had laboratory evidence of HSV infection while the rest were considered negative.
Our results corroborate very well with that of earlier investigators(11,12), who found that circulating antibody relatively remain constant, though fluctuations occurred occasionally in herpes infections. Lennette and Allen(13) observed that recurrent ocular HSV attacks are not associated with changes in the antibody level before, during or after the episodes. Similar observations were also made by Wilhelmus et al(14) also. It is well established that HSV remain latent in the neurons after the primary infection and forms an endogenous source of infection. It can repeatedly become active and give rise to recurrent infections. It is also postulated that virus give a constant or intermittent antigenic stimulus which keep the circulating antibody at a high level(15). A fluctuation FROM this level occur only in a few cases (16). These fluctuations may even occur without the evidence of active infection (17,18,19) However, Peter Fox et al (10) observed significantly higher IgG level in dendritic and conjunctival GROUP compared with other clinical groups and controls. Interestingly, our analysis of antibody titres versus type of clinical lesion showed the highest quantity of HSV IgG to be in SPK cases. The number of cases of SPK included in our study was small compared to other groups. However, this results raise interest to follow-up the study with more number of SPK cases and an equal number of cases in all the different clinical groups.
This study through the light on the status of usefulness of anti-HSV IgG serology in herpes keratitis cases. Firstly, in majority of the cases, there was no rise in antibody titre. However, in 17.85% of the cases, we saw an elevation in HSV IgG. Secondly, in 39 out of 56 (69.6%) of the seropositive cases, there was a fall in the level of IgG after the treatment, which was concurrent with the resolution of the lesion. Finally, the virus antigen detection FROM corneal scrapping is very useful to confirm the presence of HSV in keratitis patients. This way it was possible to detect HSV in one-fourth of the cases.
While the host factors involved in the recurrence and recovery remain obscure the humoral response do not appear to have any protective role. Further, it is postulated that as the specific antibodies combine to the Fc receptor of the HSV infected cell, the virus spread can occur by direct contact between cell to cell. Cell mediated immunity and interferons and other cytokines may be more important than the humoral response. Further work remains to determine the actual role of humoral response in pathogenesis and recurrence of herpes keratitis. | | | Acknowledgements | | Authors acknowledge with thanks the financial assistance received FROM the Council for Scientific and Industrial Research (CSIR), Govt. of India, New Delhi, in the form of fellowship and contingency grant. | | | References | 1) Darougar, S., Wishart, MS., Viswalingam, ND. Epidemiological and clinical features of primary HSV ocular infection. Br J Ophthalmol 69: 2 (1985)
2) Dawson, CR., Togni, B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol 21: 121 (1976)
3) Easty, DL., Cartier, C., Funk, A. Systemic immunity in herpes keratitis. Br J Ophthalmol 65: 82 (1981)
4) Friedman, MG., Kimmel, N. HSV specific immunoglobulinA: detection in patients with primary and recurrent herpes infections and in healthy adults. Infec Immun 37: 374 (1982)
5) Goodman, JL. Infections caused by HSV. In Hoeprich PD, Jordan C, Ronald AR eds: Infectious diseases ed5, Philadelphia, 1994, JB Lippincott
6) Nahmias, AJ., Roizman, B. Infection with HSV 1 and 2. N Engl J Med 289: 667 (1973)
7) Kaye, SB., Madan, N., Dowd, TC. Ocular shedding of Herpes Simplex Virus. Br J Ophthalmol 74: 114 (1990)
8) Herrmann, EC Jr. Experiences in laboratory diagnosis of Herpes Simplex, Varicella and vaccinia virus infections in routine medical practice. Mayo Clin Proc 42: 744 (1967)
9) Boerman, RH., Peters, ACB., Verheij, M. Immunofluorescence detection of Herpes Simplex type I antigen in cerebrospinal fluid cells of experimentally infected mice. J Neurol Sci 87: 245 (1988)
10) Peter, DF., Peng, TK., Brian, WM., James, IM., Kevin, AW. Tear and serum antibody levels in ocular herpetic infection: diagnostic precision of secretory IgA. Br J Ophthalmol 70: 584 (1986)
11) Jawertz, E., Allede, MF., Coleman, VR. Studies on herpes simplex virus. The level of neutralizing antibodies in human sera. J Immunol, 68: 655 (1952)
12) Wu, JS., Yamanishi, R., Kaneko, M. Serum complement-fixing antibody in herpetic ocular diseases. Nippon Ganka Gakkai Zasshi 85: 415(1981)
13) Lennette, EH., van Allen A. Laboratory diagnosis of herpetic infections of the eye. Am J Ophthalmol, 43: 118 (1957)
14) Wilhelmus, KR., Darougar, S., Forsey, T., Treharne, JD. Sequential antibody changes following ulcerative herpetic keratitis. Br J Ophthalmol 70: 354 (1986)
15) Burnet, FM., Williams, SW. Herpes Simplex : A new point of view. Med J Aust i: 637 (1939)
16) Doughlas, RG., Couch, RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol 104: 289 (1970)
17) Centifanto, Y., Little, J., Kaufman, H. Relationship between virus chemotherapy secretory antibody formation and recurrent herpetic disease. Ann NY Acad Sci,173: 649 (1973)
18) Meyers, R., Chitjian, P. Immunology of herpes virus infections: immunity to HSV in eye infections. Surv Ophthalmol 21: 194 (1976)
19) Meyers Elliot, R., Pettit, T., Maxwell, W. Viral antigens in immune ring of herpes simplex stromal keratitis. Arch Ophthalmol, 98: 897 (1980) | | | Tables |
"0">
|
Type of lesion |
Number. |
Percent |
Epithelial (n=115) |
|
|
Dendritic |
92 |
39.3 |
Geographic |
20 |
8.5 |
SPK |
23 |
9.8 |
Pseudodendritic |
12 |
5.1 |
Epithelial defect |
6 |
2.6 |
Stromal (n=35) |
|
|
Disciform |
66 |
28.2 |
Necrotizing keratitis |
15 |
6.4 |
Table 1 : Clinical presentations of the cases included
in the study (n=234)
CELLPADDING="7" CLASS="copy">
|
Type of Lesion |
Total No. collected |
No. Positive by culture and or IF |
Dendritic |
92 |
40 |
Geographic |
20 |
2 |
Superficial punctate keratitis |
23 |
4 |
Disciform |
66 |
12 |
Necritizing stromal keratitis |
15 |
3 |
Pseudodendritic |
12 |
2 |
Epithelial defect |
6 |
2 |
Table 2: Clinical lesions in all the 234 cases and its correlation with positive diagnosis.
Test |
Number (%) |
Either HSV isolation or IF positive |
65 (27.77) |
HSV isolation Positive |
36 (15.38) |
IF Positive |
62 (26.49) |
Both HSV isolation and IF positive |
32 (13.67) |
IF Positive but HSV isolation negative |
30 (12.82) |
HSV isolation Positive but IF negative |
03 (01.28) |
Table 3: HSV positivity pattern and correlation between Virus isolation & indirect IF in HSK (n=234). Mc Nemar's test (indirect IF v/s VI),P<0.05 |
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in