|
|
 |
 |
 |
 |
|
|
Age-Dependent Visual Field Loss in RCS Rats and the Relative Benefits of Subretinal Over Intravitreal RPE Transplantation
Digital Journal of Ophthalmology 1999
Volume 5, Number 7
December 15, 1999
|
Printer Friendly
|



Yves Sauvé | Institute of Ophthalmology,London,UK Henry Klassen | Institute of Ophthalmology,London,UK Simon J.O. Whiteley | Institute of Ophthalmology,London,UK Raymond D. Lund | Institute of Ophthalmology,London,UK
|
|
|
| Abstract | Objective
The consequences of progressive retinal degeneration on central visual function were studied by recording single and multi-unit receptive fields (RFs) across the surface of the superior colliculus (SC) of pigmented dystrophic RCS rats. Retinal morphology was used to provide a correlation between function and histological appearance. In addition, the potential protective effect of retinal pigment epithelium (RPE) transplantation was studied in a similar manner in dystrophic animals in which RPE cells were injected INTO the subretinal space, or the vitreous, at between 21 and 28 days of age. The visual responsiveness of SC units in dystrophic rats differed markedly FROM those in non-dystrophics. Dystrophic rats developed a relative scotoma beginning in the central visual field by 42-45 days of age and expanding to include 50% of the visual field by 97-107 days. In contrast, following subretinal RPE transplantation, there was a substantial photoreceptor rescue correlated with a partial to complete preservation of RF representation when examined at 85 to 108 days of age. Major photoreceptor rescue occurred in the region of graft placement with possible low level rescue across much of the central retina. Dystrophic animals that had received intravitreal RPE transplants showed poor photoreceptor survival as well as minimal functional preservation. Our results indicate that there is a progressive central to peripheral loss of visual responsiveness in the SC of dystrophic RCS rats which can be delayed by subretinal injections of healthy RPE cells.Keywords
retinal degeneration, RCS rat, superior colliculus, electrophysiology, retinal pigment epithelium, retinal transplantation, visual field | | | Introduction | The Royal College of Surgeons (RCS) rat has been studied extensively as a model of retinal degeneration. In this rodent a recessive mutation renders the retinal pigment epithelium (RPE) incapable of properly processing shed rod outer segments (ROS). Accumulation of debris in the subretinal space is accompanied by progressive photoreceptor cell (PRC) death [1-3]. By postnatal week 10 only a few photoreceptor cells remain; this sequence of cell death can be limited by grafts of normal RPE cells to the subretinal space made within the first month of age [4,5].
Although the anatomical features of the retinal degeneration occurring in the RCS rat have received considerable attention, little is known of the corresponding functional losses beyond effects on the electroretinogram (ERG) [6-9] and simple reflex responses [3,10,11]. Functional studies of RPE transplantation in this animal have been limited to the evaluation of responses following whole eye stimulation [11-13]. The potential benefits of RPE cell transplantation in maintaining visual function deserve more extensive investigation. Here we examine the electrophysiological correlates of photoreceptor degeneration, and preservation, as recorded in the superior colliculus (SC) of RCS rats. Since the majority of RGCs project to the contralateral SC in a topographic manner, this organization permits investigation of the functional changes occurring across the visual field. Most important is to examine how the visual field defect develops as the dystrophy progresses. In addition, the degree to which transplants serve to preserve function at a local level has also been examined. Preliminary communications of some of these results have been given [14,15]. | | | Materials and Methods | Animals
All animal care and handling during the course of this study conformed to the requirements of the British Home Office.
RCS rats were bred and maintained in our own colony. Dystrophic animals were pigmented (RCS rdy- p+) while non-dystrophic animals were congenic pigmented rats (RCS rdy+ p+). The baseline study of tectal physiology used 3 non-dystrophic RCS rats (22, 98 and 325 days) and 8 dystrophic RCS rats (23, 42, 43, 45, 97, 100, 103, and 107 days). For transplantation studies, 11 dystrophic RCS rats age 21-28 days, received subretinal or intravitreal injections of RPE cells; they were subsequently tested between 85 and 108 days. Four dystrophic rats received subretinal injections. In an additional 7 animals, the retina was perforated and the cells injected INTO the vitreous. These animals served as a comparison group.
Electrophysiology
Animals were anesthetized with a single intraperitoneal dose of urethane (1.25 g/kg).The head was held by a nosebar and the test eye immobilized with subconjunctival sutures secured to the fixation apparatus. Topical tropicamide (1.0%) was applied to provide mydriasis and a non-corrective contact lens protected the cornea. The SC contralateral to the experimental eye was surgically exposed and covered with mineral oil. The atlanto-occipital membrane was incised to attenuate respiration-related pulsations of the brain. Body temperature was monitored with a rectal probe and maintained at 37°C using a heating blanket.
A ganzfeld consisting of a translucent hemisphere (55cm radius) was centered on the test eye. Animals underwent one hour of dark adaptation at 0.34 cd/m2 prior to recording. Single and multi-unit recordings were made FROM the superficial layers of the SC to a depth of 200µm using glass-coated carbon fiber electrodes (resistance: 0.5 - 5.0 megohm, bandpass: 500 Hz - 5 Khz). Recording sites covered the full extent of the SC along a rectilinear grid of 200 µm steps. At sites where a response to whole eye illumination was found, an attempt was made to identify units responsive to stimulation of discrete RFs. The visual field was systematically searched by moving spots of light across the surface of the ganzfeld (background luminance kept at 0.34 cd/m2). Once a receptive field was defined, static stimuli (diameter: 10°, luminance: 5.7 cd/m2) were presented to its center. Presence or absence of responsiveness to standard RF illumination could then be confirmed by assembling post-stimulus time histograms (5 msec bins) over 30 consecutive stimuli. Care was taken to minimize adaptive changes due to repetitive stimulation by adjusting the inter-stimulus interval to between 1 and 20 secs.
Electrophysiological testing was performed in a blind manner, with animals FROM different groups interspersed.
Transplantation
Donor pigment epithelial cells were harvested FROM eyes of Lister Hooded rats (age: 56 to 70 days). Donors were perfused through the heart with phosphate buffered saline while under terminal anesthesia. The eyes were enucleated and the RPE dissociated following the method of Chang et al. [16]. Recipient animals were 11 dystrophic RCS rats (age: 28 days). Under anesthesia (tribromoethanol: 230 mg/kg), the head was secured by a nosebar, the sclera incised with a microsurgical scalpel and the donor cells injected INTO the subretinal space (n=5) or through the retina INTO the vitreous (n=7) using a fine glass micropipette attached to a Hamilton syringe (2 X 104 cells/µl X 2µl) [11].
Histology
At the conclusion of the recording session, the animals were perfused through the heart with 4% paraformaldehyde. A ligature was placed through the superior rectus muscle to preserve orientation and the eye was enucleated. The cornea was perforated, the eye immersed in 4% paraformaldehyde, and refrigerated at 4°C. After fixation, the anterior segment was removed using a circumferential incision through the ciliary body. The dorsal point of each eye cup was scored with a small, full thickness incision for reference prior to removal of the superior rectus. Cryoprotection in 30% sucrose was followed by embedding in OCT and storage at –70°C. Eyes were sectioned in the horizontal plain by cryostat (thickness: 14 µm) and stained with cresyl violet.
Anatomical Analysis
Histological sections FROM all eyes were examined microscopically to assess the degree of photoreceptor preservation. In a rat that had received a subretinal RPE injection, and in a second one in which the injection was INTO the vitreous, an extensive quantitative evaluation of anatomical rescue was undertaken in which the thickness of the retinal layers was measured at 200X magnification using camera lucida technique. Beginning at the optic nerve, measurements were made at 500 µm intervals along the outer retina. This process was repeated for all sections. DATA were then reconstructed as a rectilinear grid representing the full extent of the retina (total number of loci per retina: 178-195).
The retinal sampling interval of 500 µm was chosen to correspond with the intervals used for electrophysiological recording FROM the SC. DATA FROM our preparation showed that 200 µm along the surface of the SC corresponds with approximately 12° of visual space. Our calculations of the appropriate retinal interval were based on this data, a retinal diameter of 8500 µm, and DATA indicating that the retina of the rat occupies 200° of visual space [17]. | | | Results | Non-Dystrophic RCS Rats
Electrophysiological recordings FROM the SC of non-dystrophic rats showed brisk responses to projected spots of light, either turned on or off within the receptive field or moved across it (standard RF illumination: 5.7 cd/m2). A systematic mapping of the visual field representation over the extent of the SC was achieved by recording FROM adjacent points at 200 µm intervals (Fig. 1). As long as responses were recorded FROM the superficial SC, receptive field sizes were quite restricted, of the ORDER of 5-15°.
Dystrophic RCS Rats
In the SC of dystrophic rats an uninterrupted field map was obtained at 23 days of age. Animals examined at ages of 42, 43 and 45 days did not have definable RFs in the central visual field using standardized illumination. This region, referred to as the relative scotoma, corresponded to 1-9% of the total number of recording sites per animal. The number of sites not associated with a RF were 2, 1 and 7 respectively. With higher luminance, however, units within this relative scotoma still had definable RFs at incremental thresholds of 1 to 1.5 log units (Fig. 1). The loss of response to focal stimulation at standard luminance levels occurred despite the fact that the outer nuclear layer was still several cells thick in the corresponding region of the retina. In subsequent weeks the relative scotoma expanded first INTO the ventral field and then across the whole field. In animals examined at ages of 97, 100, 103 and 107 days of age, there was a relative scotoma involving 13-34% of the ventrocentral VF. The number of sites not associated with a RF were 12, 13, 29 and 22 respectively, typically 50° across (Fig. 1). Again, responsiveness to focal stimulation could be obtained within the relative scotoma at higher luminance levels.
In all areas of the SC outside of the relative scotoma, visual receptive fields were appropriately arrayed: their position was not affected by the presence of an adjacent area devoid of discrete input using standard illumination.
RPE Cell Transplantation
Animals that had received RPE injections were tested for responsiveness to RF illumination at 85-108 days of age. Some of these had received injections INTO the subretinal space (n=4) and some INTO the vitreous (n=7). The number of recording sites for which RFs could not be defined was used to quantify the size of the scotoma in each animal. A statistical comparison was made between these two groups and age-matched non-transplanted dystrophics (n=4); Kruskal-Wallis with 3 levels: no transplantation, subretinal RPE injection, and intravitreal RPE injection (Fig. 4; see TABLE 1 for individual data).
In the subretinal RPE injection group, the scotoma size was significantly smaller than in either other group; p=0.0185 (Fig. 4). Figure 2 shows 1 of the 3 cases in which the expected development of a relative scotoma did not occur following subretinal RPE transplantation. At the light microscopic level, for all 4 animals with subretinal injections, there was indication of photoreceptor rescue as assessed by apparent ONL integrity, PRC density, and presence of a zone of rod inner and outer segments. This was also confirmed using quantitative measurements of ONL thickness in one animal for which no scotoma was detected (Fig. 2).
Topographically, a relatively circumscribed area of maximal PRC preservation was evident that corresponded in approximate size and location to the original subretinal injection. Furthermore, a well-defined layer of outer and inner segments was only found in the area of greatest rescue that was centered on the original injection site. Beyond the locus of transplantation there was a peripheral rim of ONL sparing, also seen in age-matched controls, which was relatively thick but the ONL nuclei appeared to be more widely spaced indicating decreased PRC density. There was no layer of photoreceptive elements visible in these areas. In addition, a low level of diffuse rescue appeared to be present centrally which was not found in age-matched controls. This might account for the lack of a relative scotoma seen in 3 out of the 4 animals with subretinal RPE injections.
There was no significant reduction of the scotoma size for the intravitreal RPE injection GROUP (Fig. 3) when compared with age-matched untreated dystrophic rats (Fig. 4).
In the best case among this group, the partial functional rescue was associated with prominent focal anatomical rescue near the injection site. Notably, there was some evidence of rounded-up pigmented cells in the subretinal space adjacent to the injection site, suggesting a subretinal component to the injection. Exhaustive anatomical investigation of one of these animals (for which 9 sites were not associated with a RF) showed limited photoreceptor preservation restricted around the injection site, without evidence of low level rescue across the retina (Fig. 3). | |
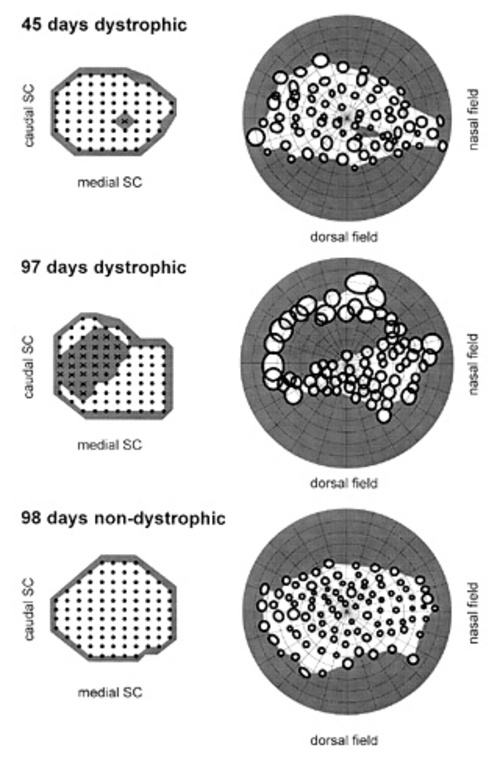
Figure 1
Progression of a relative scotoma with age in dystrophic RCS rats and comparison with visual field in old non-dystrophic RCS rat. (A) 45 days dystrophic; (B) 97 days dystrophic; (C) 98 non-dystrophic . On right, position of SC units associated with RFs (solid circles), not associated with RFs (X), and non visually responsive (small dots). On left, visual RFs corresponding to the SC units. Grey areas indicate the extent of the relative scotoma. Note that RFs are defined using standardized illumination (5.7 cd/m2 spots on 0.34 cd/m2 background).
|
|
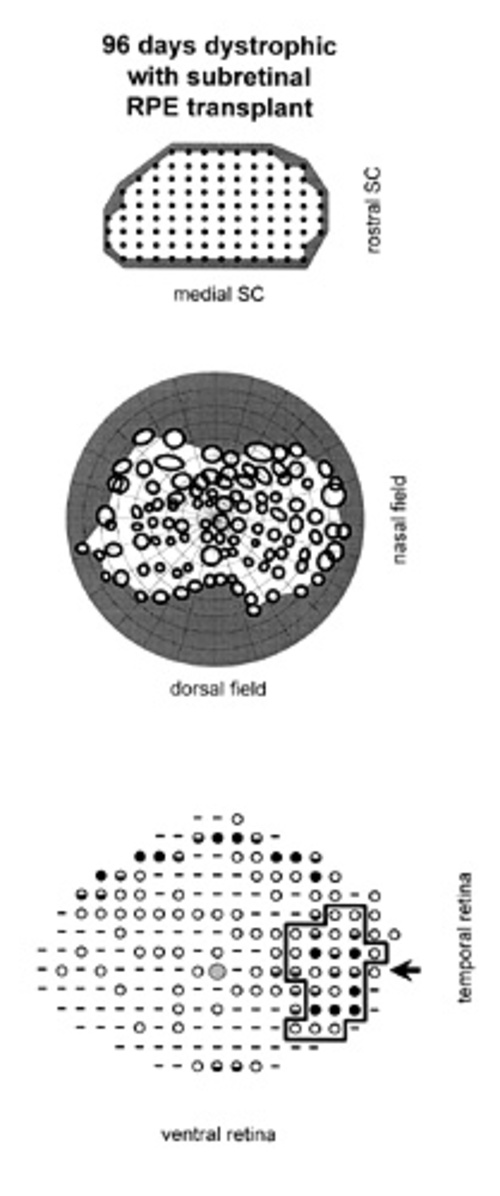
Figure 2
Case in which RPE cells were injected INTO the subretinal space. Correlation of visual function with anatomical rescue: all maps are presented in corresponding orientations. (A) Electrophysiological recording sites in the superior colliculus. In this animal, units with definable visual receptive fields were found at sites tested (solid circles). (B) The receptive fields, corresponding to the SC recording sites shown above, are plotted across the visual field. No relative scotoma was present. (C) Outer nuclear layer thickness measured across the retina. Rescue is broadly spread FROM the area of the injection. Dashes indicate a thickness of 0-1 nuclei, open circles=2-3 nuclei, half-filled circles=4-5 nuclei, solid circles=6 or more nuclei. The large hatched circle represents the position of the optic disc. The arrow points toward the injection site. The bold outline encloses the retinal area exhibiting a well-defined layer of rod photoreceptive elements, perceptible at the light microscopic level.
|
|
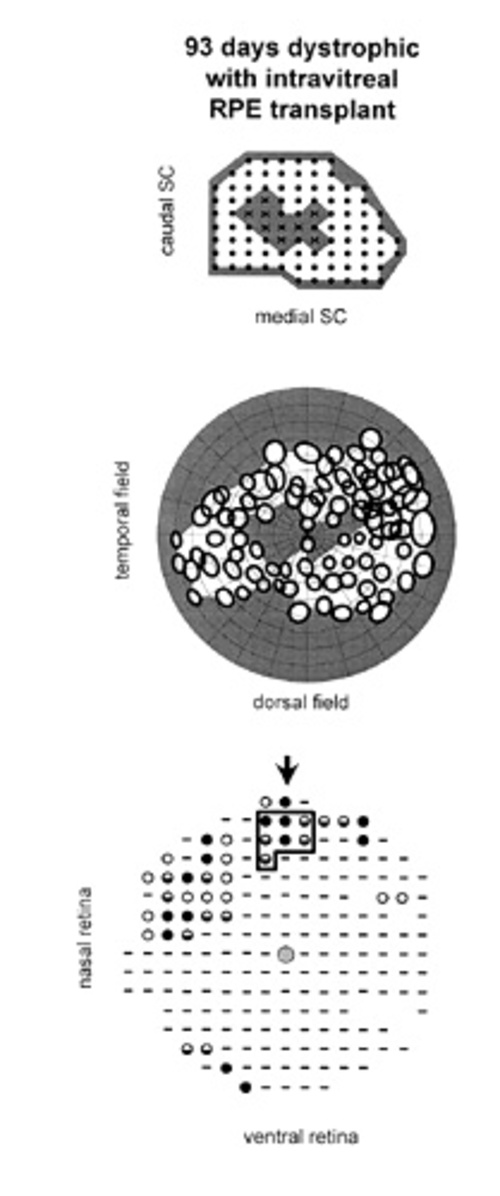
Figure 3
Case in which RPE cells were injected INTO the vitreous. Correlation of visual function with anatomical rescue: all maps are presented as in Fig. 2. (A) Collicular electrophysiology. In this animal, units with definable receptive fields could not be found in the central colliculus (X) using standardized luminance levels. (B) The resulting relative scotoma is seen in the central visual field. (C) Outer nuclear layer thickness is increased focally around the injection site, but is more limited than in Fig. 2.
|
|
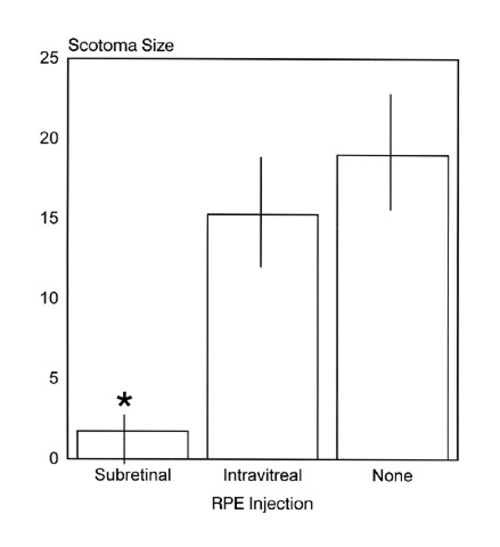
Figure 4
Comparison of the scotoma size for each experimental GROUP of age-matched dystrophic RCS rats: subretinal RPE injections, intravitreal RPE injections, and untreated. The size of the scotoma is expressed as the number of recording sites for which no visual receptive field could be mapped. All groups were compared using a Kruskal-Wallis test with 3 levels. There was a significant reduction in scotoma size for the subretinal RPE injection GROUP (*) when compared with the two other groups (p=0.0185). There was no significant difference in scotoma size between animals that did not receive RPE injections and ones in which RPE cells were injected INTO the vitreous. Bins represent the mean scotoma size and error bars the standard deviation.
|
|
| Discussion | This study uses the retino-collicular pathway to investigate how the retinal degeneration of the RCS rat gives rise to a visual field defect and how this process is affected by transplantation of RPE cells INTO either the subretinal space or the vitreous cavity.
The development of the visual field defect in the dystrophic RCS rat is a slow process occurring over a period of more than 6 months. A relative scotoma first appears in the central VF at about day 40 and expands progressively to cover the entire VF at around day 200. During this period visual responses can still be recorded within the relative scotoma, but with increases in threshold of between 1 and 4 log units. By 400 days the scotoma becomes absolute over the entire retina. In previous studies, recordings of ERGs FROM RCS rats have also indicated a progressive elevation of threshold with age [7,9,18]. However, the disappearance of the a-wave (photoreceptor-related component) by 50 days of age and of the b-wave by 100 days in pigmented dystrophic RCS rats [9] precludes examination of visual sensitivity using this test at later degenerative stages. Other approaches involving recordings FROM the optic tract [19] and the visual cortex [20] have clearly demonstrated visual responsiveness to whole eye illumination beyond 150 days in the RCS rat. It therefore appears that recordings FROM RGC axons or CNS visual centers are more sensitive than ERGs in detecting the retina's continued capacity for phototransduction. Combining this former approach with RF mapping makes it possible to assess function at specific points across the retina.
The present retinotopic mapping in the superior colliculus of the RCS rat, as well as similar mapping in the rd mouse [21], do correlate to some extent with the pattern and rate of anatomical loss. For instance, the loss of receptive fields in the rd mouse also occurs in a central to peripheral gradient, although it already covers 90-100° of the VF by postnatal day 24. This is in agreement with the more rapid anatomical degeneration in the rd mouse as compared to the RCS rat. The first evidence of field loss in the RCS rat occurs in the central retina where the photoreceptor layer is still at least 5 cells thick. Yet at later times, in areas of the retina FROM which discrete receptive fields can still be localized, the photoreceptor layer is only 1-2 cells thick. This raises an issue already addressed in studies of the pupilloconstrictor response in which density of photoreceptors alone is not always predictive of visual function [11,22]. Other work on the RCS rat has found upregulation of c-fos activity in the superior colliculus [23], which may reflect changes in ganglion cell activity. In accord with this we have found increased spontaneous activity in collicular units in dystrophic RCS rats while a similar phenomenon has been reported for recordings FROM RGCs [12], their axons [19] and the visual cortex [20]. It would appear therefore that the degenerative events are accompanied by compensatory mechanisms, some of which appear to occur in the retina, which may allow the CNS to deal effectively with a reduced sensory signal.
Our results indicate that transplantation can have a direct and effective influence on the deterioration of visual responsiveness and there is a good correlation between the area of PRC preservation and the region demonstrating functional preservation. Within the area of functional rescue, appropriate retinotopy is preserved. Clearly this issue should be examined in more detail at longer survival times where a region with preserved function might be surrounded by retinal areas FROM which focal responses can no longer be elicited. This is important to consider because there is evidence in both somatosensory and visual cortex of plasticity of regional representations after focal peripheral lesions [24]. There is, however, a major difference here in experimental design. Apart FROM the fact that the lesion is several synapses removed FROM the retinal output, there is also a signal coming FROM the relative scotoma albeit with a much higher visual threshold. In those animals in which RPE cells were injected INTO the vitreous, there is local rescue around the injection site which can be seen both anatomically and physiologically. Interestingly, in these animals, there is little indication of more wide spread trophic influences that might be anticipated if the grafted cells were providing a necessary diffusible factor [25].
This study assumes further importance as transplantation experiments are considered in humans [26] and provides a potential source of comparison between animal and human studies, particularly with respect to visual field assessment. Clearly, however, to be able to say whether the rat shows visual improvements, it will still be necessary to examine cortical function and associated acuity measures. Such tests will be done in coordination with behavioral studies of visual acuity [27]. | | | Acknowledgements | | This research project was funded by grants FROM the Foundation Fighting Blindness, Fight For Sight (UK), Medical Research Council (UK), and National Eye Institute (to Henry Klassen). The authors thank Dr. Teresa. Litchfield for the preparation of the RPE cells for transplantation and Tim Pheby for his skilled technical assistance. They also thank Dr. Jean Lawrence and Dr. Sergej Girman for their interest in this work and stimulating discussions. | | | References | 1. Dowling, JE., Sidman, RL. Inherited Retinal Dystrophy in the Rat. Journal of Cell Biology 14:73 (1962)
2. Bok, D., Hall, MO. The Role of Pigment Epithelium in the Etiology of Retinal Dystrophy in the Rat. Journal of Cell Biology 49:664 (1971)
3. LaVail, MM., Sidman, M., Rausin, R., Sidman, RL. Discrimination of Light Intensity by Rats with Inherited Retinal Degeneration: A Behavioral and Cytological Study. Vision Research 14:693 (1974)
4. Li, L., Turner, JE. Inherited Retinal Dystrophy in the RCS Rat: Prevention of Photoreceptor Degeneration by Pigment Epithelial Cell Transplantation. Experimental Eye Research 47:911 (1988)
5. Lopez, R., Gouras, P., Kjeldbye, H., Sullivan, B., Reppucci, V., Brittis, M., Wapner, F., Goluboff, E. Transplanted Retinal Pigment Epithelium Modifies the Retinal Degeneration in the RCS Rat. Investigative Ophthalmology and Visual Science 30:586 (1989)
6. Perlman, I. Dark-Adaptation in Abnormal (RCS) Rats Studied Electrophysiologically. Journal of Physiology 278:161 (1978)
7. Fulton, AB. Background Adaptation in RCS Rats. Investigative Ophthalmology and Visual Science 24:72 (1983)
8. Noell, WK., Pewitt, EB., Cotter, JR. ERG of the Pigmented Rdy Rat at Advanced Stages of Hereditary Retinal Degeneration. Progress in Clinical Biology Research 314:357 (1989)
9. Bush, RA., Hawks, KW., Sieving, PA. Preservation of Inner Retinal Responses in the Age Royal College of Surgeons Rat; Evidence Against Glutamate Toxicity in Photoreceptor Degeneration. Investigative Ophthalmology and Visual Science 36:2054 (1995)
10. Trejo, LJ., Cicerone, CM. Retinal Sensitivity Measured by the Pupillary Light Reflex in RCS and Albino Rats. Vision Research 22:1163 (1982)
11. Whiteley, SJO., Litchfield, TM., Coffey, PJ., Lund, RD. Improvement of the Pupillary Light Reflex of Royal College of Surgeons Rats Following RPE Cell Grafts. Experimental Neurology 140:100 (1996)
12. Yamamoto, S., Du, J., Gouras, P., Kjeldye, H. Retinal Pigment Epithelial Transplants and Retinal Function in RCS Rats. Investigative Ophthalmology and Visual Science. 34:3068 (1993)
13. Jiang, LQ., Hamasaki, D. Corneal Electroretinographic Function Rescued by Normal Retinal Pigment Epithelial Grafts in Retinal Degenerative Royal College of Surgeons Rats. Investigative Ophthalmology and Visual Science 35:4300-4309 (1994)
14. Sauvé, Y., Klassen, H., Whiteley, SJO., Litchfield, TM., Lund, RD. Functional Preservation Following RPE Grafting: I: Retinotopic Mapping in the Superior Colliculus. ARVO Abstracts. Investigative Ophthalmology and Visual Science 37:S93 (1996)
15. Sauvé, Y., Klassen, H., Whiteley, SJO., Litchfield, TM., Lund, RD. Retinotopic Analysis of Response Parameters in the Superior Colliculus of RCS Rats Following Retinal Pigment Epithelial Cell Transplantation. Society of Neuroscience Abstracts. 22:1978 (1996)
16. Chang, CW., Roque, RS., Defoe, DM., Caldwell, RB. An Improved Method for Isolation and Culture of Pigment Epithelial Cells. Current Eye Research. 10:1081 (1991)
17. Hughes, A. The Topography of Vision in Mammals of Contrasting Life Style: Comparative Optics and Retinal Organization. In: Crescitelli, ed. Handbook of Sensory Physiology. Berlin:Springer 615 (1977)
18. Deshpande, S., Thompson, M., Parker, JA., Abrahamson, EW. Study of Retinal Dystrophy in RCS Rats: A Comparison of Mg-ATP Dependent Light Scattering Activity and ERG B-Wave. Vision Research 32:425 (1992)
19. Cicerone, CM., Green, DG., Fisher, LJ. Cone Inputs to Ganglion Cells in Hereditary Retinal Degeneration. Science 203:1113 (1979)
20. Noell, WK., Salinsky, MC. Preservation of Visual Evoked Cortical Responses at Advanced State of Retinal Degeneration in the Rdy Rat. In: LaVail, MM., Hollyfield, JG., Anderson, RE., eds. Retinal Degeneration. New York: Alan R Liss 301 (1985)
21. Dräger, UC., Hubel, DH. Studies of Visual Function and its Decay in Mice with Hereditary Retinal Degeneration. Journal of Comparative Neurology 180:85 (1978)
22. Kovalevsky, G., DiLoreto, D. Jr., Wyatt, J., del Cerro, C., Cox, C., del Cerro, M. The Intensity of the Pupillary Light Reflex Does Not Correlate with the Number of Retinal Photoreceptor Cells. Experimental Neurology. 133:43 (1985)
23. Lu, B., Coffey, PJ., Lund, RD. C-Fos Expression in Retina and Superior Colliculus of RCS Rat Following Light Flashes. ARVO Abstracts. Investigative Ophthalmology and Visual Science 37:S1047 (1996)
24. Gilbert, CD., Wiesel, TN. Receptive Field Dynamics in Adult Primary Visual Cortex. Nature 356:150 (1992)
25. Faktorovich, EG., Steinberg, RH., Yasumura, D., Matthes, MT., LaVail, MM. Photoreceptor Degeneration in Inherited Retinal Dystrophy Delayed by Basic Fibroblast Growth Factor. Nature 347:83 (1990)
26. Algvere, PV., Berglin, L., Gouras, P., Sheng, Y. Transplantation of Fetal Retinal Pigment Epithelium in Age-Related Macular Degeneration with Subfoveal Neovascularization. Graefes Archives of Clinical and Experimental Ophthalmology 232:707 (1994)
27. Coffey, PJ., Hetherington, L., Binn, L., Whiteley, SJ., Litchfield, TM., Lund, RD. Detection of Visual Patterns and C-Fos Expression in Retina and Superior Colliculus of Dystrophic RCS Rats Following RPE Transplants. ARVO Abstracts. Investigative Ophthalmology and Visual Science 37:S94 (1996) | | | Tables |
Table 1: Scotoma size for each experimental group
of age-matched dystrophic RCS rats
Subretinal RPE injection |
Intravitreal RPE injection |
No injection |
|---|
0 |
4 |
12 |
0 |
9 |
13 |
0 |
12 |
22 |
7 |
18 |
29 |
|
19 |
|
|
19 |
|
|
27 |
|
Individual values of scotoma size for each experimental group
of age-matched dystrophic RCS rats: subretinal RPE injections,
intravitreal RPE injections, and untreated. The size of the scotoma
is expressed as the number of recording sites for which no visual
receptive field could be mapped. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in