|
|
 |
 |
 |
 |
|
|
Elevated TIMP-3 Levels in Bruch's Membrane and Drusen in Eyes FROM Age-Related Macular Degeneration Donors
Digital Journal of Ophthalmology 1998
Volume 4, Number 11
December 25, 1998
|
Printer Friendly
|



Motohiro Kamei | The Cleveland Clinic Foundation, Cleveland, OH, USA Mary E Rayborn | The Cleveland Clinic Foundation, Cleveland, OH, USA Suneel S Apte | The Cleveland Clinic Foundation, Cleveland, OH, USA Joe G Hollyfield | The Cleveland Clinic Foundation, Cleveland, OH, USA
|
|
|
| Introduction | Visual loss FROM late onset degeneration of the macula, the disorder referred to as age related macular degeneration (ARMD), is the leading cause of blindness in individuals over the age of 50 in industrialized nations [1]. ARMD begins with the accumulation of extracellular debris (drusen) within Bruch's membrane during the early stages of the disease, with the subsequent development of choroidal neovascularization or atrophy of the choriocapillaris and retinal pigment epithelium (RPE) at later stages. The latter events are thought to be causally involved in the death of macular photoreceptors. Little is known about the cellular mechanisms associated with the normal maintenance and turnover of Bruch's membrane, the events that lead to drusen formation, or the cause of subretinal neovascularization and atrophy of the RPE and choriocapillaris.
Bruch's membrane is a complex extracellular matrix located between the RPE and the endothelial cells of the choriocapillaris, and is thought to be a product of these two tissue layers which it separates. Matrix metalloproteinases (MMPs) constitute a family of enzymes which are involved in degrading components of the extracellular matrix (ECM) in the normal course of matrix turnover and renewal [2]. MMPs are also implicated during the initial stages of neovascularization, where they are thought to be required, along with other proteases, for degradation of components of the capillary basement membrane as a prerequisite for new vessel outgrowth [3]. Under normal conditions, it is thought that endothelial cells are maintained in a quiescent state because of a carefully controlled balance between angiogenic stimulators and inhibitors of angiogenesis. Regulation of the activity of the MMPs released by endothelial cells is accomplished through the inhibitory activity of a GROUP of secreted proteins referred to as TIMPs (tissue inhibitors of metalloproteinases) [4,5]. TIMPs are thought to constitutively suppress excessive degradation of the ECM and inhibit the development of neovascularization. TIMPs non-covalently bind to MMPs with high affinity which results in the inhibition of their enzymatic activity [4,5].
Mutations in the gene coding for TIMP-3 (see ref. 6 for a recent review of TIMP-3) were recently found in some families with Sorsby's fundus dystrophy, which is an early-onset inherited disease, characterized by thickening of Bruch's membrane and submacular neovascularization [7]. In situ hybridization studies indicate that the RPE is a major site of TIMP-3 expression [8,9] and immunocytochemical studies indicate that TIMP-3 immunoreactivity is present in normal Bruch's membrane [10]. Collectively, these studies suggest that the analysis of MMPs and TIMPs may be important in understanding other pathologies which affect the RPE-choroidal interface. In response to these observations, we were prompted to evaluate the levels of TIMP-3 immunoreactivity in drusen and the thickened Bruch's membrane, which are common pathological features of ARMD donor eyes. | | | Materials and Methods | The tissues used in this analysis were normal donor eyes (from two male and two female donors, 62 to 73 years of age) enucleated between 1 to 18 hours postmortem; and ARMD eyes (from four male donors between 72 and 96 years of age), enucleated between 3 and 11 hours postmortem. Normal donor tissue was obtained FROM the Cleveland Eye Bank, Cleveland, Ohio. Tissues FROM ARMD donors were obtained through the Eye Donor Program of The Foundation Fighting Blindness, Hunt Valley, Maryland. Immuno-histochemistry was performed on unfixed cryosections through the macula, using a mouse monoclonal anti-human TIMP-3 antibody [9] (Clone 136-13H4, Fuji Chemical Industries, Ltd., Toyama, Japan). The avidin-biotin method was used and immunoreactivity was resolved with horseradish peroxidase-aminoethylcarbazole (AEC) reaction product visualized with light microscopy. The reaction product produces a deep red to magenta color in the color images presented. For controls, non-immune mouse IgG was applied instead of the primary antibody.
For biochemical studies, proteins were solubilized FROM 1 cm square RPE-choroidal samples centered on the macula FROM normal and ARMD eyes. The tissues were solubilized in SDS, proteins were separated with polyacrylamide gel electrophoresis and transferred to a membrane for Western blotting with the TIMP-3 antibody. | | | Results | | Bruch's membrane in both normal and ARMD tissues showed strong immunoreactivity with the TIMP-3 antibody. In some instances, the RPE detached FROM Bruch's membrane during tissue processing (Figs. 1-2), whereas in other samples, the RPE was retained (see Figs. 3-6). In normal tissue (Figs. 1-3) Bruch's membrane usually appeared intensely stained with the magenta reaction product across the full thickness of this lamina (Fig. 2), indicating strong TIMP-3 immunoreactivity throughout this compartment. When drusen were observed in the normal tissue, they were also highly immunoreactive with the TIMP-3 antibody, and in some instances appeared more intensely labeled than the adjacent Bruch's membrane (Fig. 3). In normal control tissues incubated with non-immune mouse IgG instead of the TIMP-3 monoclonal antibody, Bruch's membrane shows no immunoreactivity and appears only as a brightly birefringent line (Fig. 2). In the ARMD tissue (Fig. 4), an extensive accumulation of near-continuous debris elevates the RPE FROM Bruch's membrane. In addition to TIMP-3 immunoreactivity in the band that represents Bruch's membrane, the accumulated debris below the RPE is also strongly immunoreactive. No immunolabeling was observed in the negative controls in either Bruch's membrane or in the debris in the ARMD tissues (Fig. 5). In several locations, TIMP-3 immunoreactivity was located beyond Bruch's membrane in finger-like extensions that surrounded choroidal vessels (Fig. 6). No TIMP-3 immunoreactivity was detected in the neurosensory retina, at more sclerad regions of the choroid, or in the sclera. When present in the frozen sections, the RPE did not SHOW immunoreactivity, although low levels of signal, if present, may have been difficult to detect because of melanin granules within the RPE cytoplasm which could mask the reaction product (Figs. 3-4). In Western blots, TIMP-3 was present in samples of both normal and ARMD tissues (not shown). | |
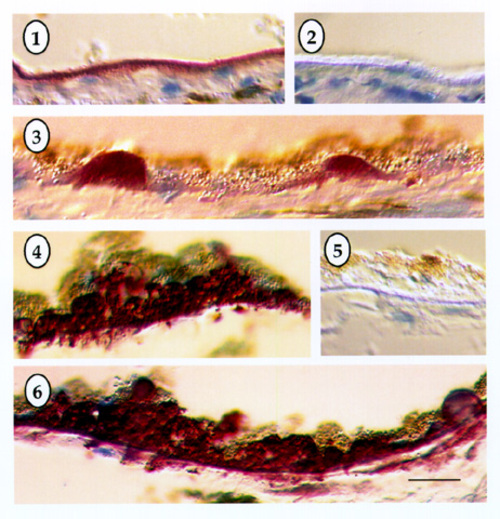
Figures 1-6
Immunohistochemistry demonstrating the localization of tissue inhibitor of metalloproteinases-3 (TIMP-3) in Bruch's membrane of normal (1-3) and age-related macular degeneration tissues (4-6). Magenta colored reaction products are present in Bruch's membrane in both normal (1-3) and ARMD tissues (4-6). The negative controls (2 and 5) SHOW a brightly birefringent Bruch's membrane free of immunolabeling. Continuous drusen accumulations are present beneath the RPE in the ARMD sections (4 and 6), which are intensely immunoreactive with the TIMP-3 antibody. Bar in lower right of Fig. 6 represents 30 µm.
|
|
| Discussion | In this analysis, we have documented the presence of TIMP-3 immunoreactivity in Bruch's membrane and drusen in both normal and ARMD human eyes. Further, there also appears to be a dramatic increase in TIMP-3 content thrroughout the accumulated debris associated with Bruch's membrane in the ARMD tissues. Studies in the mouse retina indicate that TIMP-3 mRNA expression can be detected at high levels in the RPE of normal mouse eyes [8,9]. While TIMP-3 is known to be expressed in a variety of tissues [11,12], the observation of elevated expression in the RPE suggests that much of the TIMP-3 present in Bruch's membrane may be synthesized and delivered to this extracellular site by the RPE. This is particularly likely since TIMP-3 has high affinity for the ECM and is unlikely to be soluble [13].
What role does TIMP-3 play in Bruch's membrane? The answer to this question may be provided in the future by a functional analysis of the mutations in Sorsby's fundus dystrophy, as well as further insight INTO the biochemistry of TIMP-3. Although TIMP-3 is comparable to other TIMPs in MMP inhibition [14], the basis for its binding to ECM is not known. TIMP-3 is known to be anti-angiogenic both in vitro and in vivo [15], in which respect it is similar to other TIMPs [16]. The concentration of TIMP-3 in Bruch's membrane may allow it to function as a potent local inhibitor of MMPs, preventing angiogenesis FROM the choroidal circulation.
TIMP-3 may also be involved in regulating the activity of MMPs involved in the degradation of matrix components during normal turnover and renewal of Bruch's membrane. The accumulation of debris (drusen) in Bruch's membrane in normal and ARMD tissues, may be due to an imbalance in the TIMP-MMP ratio leading to a reduction in MMP activity and an accompanying thickening of Bruch's membrane. In this context, it is crucial to identify the matrix degrading enzymes and inhibitors produced by the RPE and endothelial cells of the choriocapillaris, as well as to identify the factors regulating their expression and activity.
Because of the significance of Bruch's membrane permeability in the trafficking of metabolites between the choroid and RPE, along with the known early alterations in this lamina in ARMD, a full understanding of the regulation of turnover of the components which comprise this complex layer is needed. The highly specific localization of TIMP-3 in Bruch's membrane of normal tissues, and the apparent increased concentration of this MMP inhibitor in drusen in ARMD tissues, suggests that knowledge of the role of MMPs and TIMPs, which function in Bruch's membrane, are important areas for further study and will be needed for an understanding of several pathologies which occur at this ocular interface.
Could mutations in the TIMP-3 gene cause ARMD? It is not yet clear whether the thickening of Bruch's membrane seen in ARMD is due to a primary excess of TIMP-3 or part of a generalized accumulation of ECM. Structural mutations in the TIMP-3 gene have been sought in individuals with ARMD but have not been demonstrated thus far [17]. Sib-pair analysis has shown that there is no allele-sharing at the TIMP-3 locus in siblings with ARMD, suggesting that neither structural mutations nor mutations in regulatory regions of the TIMP-3 gene are likely to cause ARMD in the population thus far studied [18].
Given its function in MMP inhibition, its ECM binding properties, and the observations we have made in ARMD, it is likely that TIMP-3 accumulation in Bruch's membrane has substantial pathogenic relevance, even if it occurs downstream of other primary genetic defects. It is possible that there is a defect in a gene regulating the TIMP-3 promoter, or that TIMP-3 accumulates in Bruch's membrane because of a build-up of its interacting ECM partner. Whatever the mechanism, the accumulation of TIMP-3 has the potential to prevent normal remodeling of Bruch's membrane. The subsequent thickening of Bruch's membrane will likely impair retinal nutrition and function.
While these observations can only be viewed as preliminary at this time, further studies are required to determine whether additional ARMD eyes SHOW the changes we observed in this small number of samples. The role of TIMP-3 in ARMD will become more clear as investigations continue INTO its binding to ECM and INTO the molecular factors involved in its regulation. | | | Acknowledgements | | The work was supported by The Foundation Fighting Blindness, Hunt Valley, MD, the Retina Research Foundation, Houston, TX and the National Institutes of Health (Grant AR44436). We thank Dr. Bela Anand-Apte for valuable technical guidance and discussion. We gratefully acknowledge Dr. K. Iwata, Fuji Chemical Industries, Ltd., for providing the TIMP-3 antibody for these studies. | | | References | 1. Bressler NM, Bressler SB, Fine SL, 1988, Age-related macular degeneration. Surv Ophthalmol 32:375-397.
2. Birkedal-Hansen H, Moore WGI, Bodden MK, 1993, Matrix metalloproteinases : a review. Oral Biol Med 4:197-250.
3. Klagsbrun M and Folkman J, 1990, Handbook of Experimental Pharmacology 95:549-586.
4. Woessner JF Jr, 1991, Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB Letters. 5:2145-2154.
5. Murphy, G., & Willenbrock, F. 1995. Tissue inhibitors of matrix metalloendopeptidases. Methods Enzymol. 248: 496-510.
6. Anand-Apte, B., Bao, L., Smith, R., Iwata, K., Olsen, B.R., Zetter, B., and Apte, S.S. "A review of Tissue Inhibitor of Metalloproteinases-3 (TIMP-3) and experimental analysis of its effect on primary tumor growth." Biochemistry and Cell Biology, 1996, 74,853-862
7. Weber BHF, Vogt G, Pruett RC, Stohr H and Felbor U, 1994, Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP-3) in patients with Sorsby's fundus dystrophy. Nature Genetics 8:352-356.
8. Della NG, Campochiaro PA and Zack DJ, 1996, Localization of TIMP-3 mRNA expression to the retinal pigment epithelium. Invest Ophthalmol Vis Sci, 37:1921-1924.
9. Ruiz A, Peterson SS, Bok D, 1996, TIMP-3 is expressed in the human pigment epithelium. Biochem. Biophys. Res. Comm. 226:467-474.
10 . Fariss RN, Apte SS, Olsen BR, Iwata K, and Milam AH, 1997, Tissue inhibitor of metalloproteinases-3 is a component of Bruch's membrane of the eye. Am J Path 150:323-328.
11. Apte SS, Hayashi K, Seldin MF, Mattei M-G, Hayashi M, Olsen BR, 1994, The gene encoding a novel murine tissue inhibitor of metalloproteinases (TIMP), TIMP-3, is expressed in epithelium, cartilage and muscle during development, and is located on chromosome 10. Developmental Dynamics, 19:86-90
12. Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR, 1994. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 269: 9352-60.
13. Pavloff N, Staskus P, Kishnani NS and Hawkes SP, 1992, A new inhibitor of metalloproteinases FROM chicken: ChIMP-3. A third member of the TIMP family.. J Biol Chem. 267:17321-17326.
14. Apte SS, Olsen BR and Murphy G, 1995, The gene structure of tissue inhibitor of metalloproteinases (TIMP-3) and its inhibitory activities define the DISTINCT TIMP gene family. J Biol Chem. 270:14313-14318.
15. Anand-Apte B, Pepper MS, Voest E, Montesano R, Olsen BR, Murphy G, Apte SS, Zetter B, 1997, Inhibition of angiogenesis by tissue inhibitor of metalloproteinases-3. Invest Ophthalmol Vis Sci. 38: in press.
16. Moses MA and Langer R, 1991, A metalloproteinase inhibitor as an inhibitor of neovascularization. J Cell Biochem. 47:230-235.
17. Felbor U, Doepner D, Schneider U, Zrenner E, Weber BHF, 1997, Evaluation of the gene encoding the tissue inhibitor of metalloproteinases-3 in various maculopathies. Invest. Ophthalmol. Vis Sci. 38:1054-1059.
18. De La Paz, M, Pericak-Vance, MA, Lennon, F, Haines, JL and Seddon, JM. Exclusion of TIMP3 as a candidate locus in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 38:1060-1065. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in