|
|
 |
 |
 |
 |
|
|
Development in Retinal Cell Transplants
Digital Journal of Ophthalmology 2001
Volume 7, Number 2
April 1, 2001
|
Printer Friendly
|



R.K. Sharma | University of Lund, Lund, Sweden Fredrik Ghosh | University of Lund, Lund, Sweden A. Bruun | University of Lund, Lund, Sweden K. Arner | University of Lund, Lund, Sweden B. Ehinger | University of Lund, Lund, Sweden
|
|
|
| Abstract | Methods
Neural retinas FROM embryonic day 15 outbred pigmented rabbits were used as donor tissue. Adult rabbits of the same strain weighing 1-2.5 kg, were used as recipients. The donor tissue was drawn up in a thin polyethylene capillary mounted on a special instrument and connected to a precision microsyringe. The capillary then contained fragmented pieces of both peripheral and central donor tissue mixed together. The recipient eye was entered through a small scleral incision 2-4 mm behind the limbus. The capillary was advanced through the vitreous to the posterior pole of the eye, and the plastic capillary was then pushed INTO the subretinal space where its content was deposited. No antibiotics or immunosuppressive drugs were used on the animals. The details of the procedure have been published [15].
The transplants were studied at developmental ages corresponding to embryonic day (E) 16 to post natal day (PN) 107. The distribution of the Ki67 antigen was examined in cryostat sections of the retinas, applying standard immunohistochemical methods and the MIB-1 monoclonal antibody (Immunotech, Inc., Westbrook, ME) in a dilution of 1:200 in PBS.
For transplants we will use the term "luminal layers" or "inner layers" to denote the layers of cells towards the lumen of the rosettes and "outer layers" to denote layers away FROM the lumen. For developing retinas, the term "basal" and "apical" is used to denote localization in terms of how the tissue develops. In the neuroretina, "basal" thus means closer to the vitreous and "apical" closer to the photoreceptors.
Results
One day after the transplantation (i. e. at E16), rosettes had started to form in the transplants. Ki67 immunoreactive cells were observed scattered throughout the transplant. Certain large and deeply stained cells were homogeneously distributed throughout the transplant or occasionally formed small rosette-like clusters (fig. 1).
The size, shape and staining characteristics of the deeply stained cells were identical to those in the late phases of mitosis, described in the normal retina [24,25]. Immunoreactive and non-immunoreactive cells were homogeneously distributed throughout the transplant. On rare occasions, a few cells close to the host retina formed small, thin patches of small and pyknotic cells devoid of immunoreactivity. The host retina also showed small and pyknotic cells. No immunoreactive cells were seen in the host retina [23].
In E 19 transplants, the cells lying close to the host retina were small and pyknotic and devoid of immunoreactivity [23]. But a few immunoreactive cells were seen at the host graft interface, which persisted even in older ages as will be noted below. A few immunoreactive cells were seen in the host retina [23]. Pyknotic or non-immunoreactive cells were not observed at the sides of the transplants, even though they were still close to the host retina. Similarly small transplants covered with the host retina lacked non-reactive pyknotic cells (Fig 2).
By E 21 or 22 , the rosettes were more distinct. Small and pyknotic cells were still visible. Large, deeply immunoreactive mitotic cells were often found arranged in the luminal layers of the rosettes [23]. Certain cells in the luminal layers of the rosettes had started becoming non-reactive., but the deeply stained cells were still present. The reactive cells were located in the outer layers of the rosettes and also in between the rosettes The layer of non-reactive, pyknotic cells close to the host retina persisted as described above.
By E 26 (fig. 3), most cells, except the large, deeply staining cells, were non-reactive in the inner layers of the rosettes. Reactive cells were located in the outer layers of the rosettes and in between the rosettes. Some immunoreactive cells were present in the host retina.
At E 29, cells in the inner layers of the rosettes were non-reactive, with the exception of certain large deeply reactive mitotic cells (fig. 4). However, cells in the outer layers of the rosettes and in between them were immunoreactive (fig. 4). On the graft side of the host-graft interface, there was a concentration of immunoreactive cells.
At PN 2, the cells in between the rosettes were still immunoreactive and there were immunoreactive cells in the host retina (Fig 5) and host-graft interface (fig. 5). At a PN 4, most of the transplants were devoid of immunoreactive cells, except occasional patches in between the rosettes. Immunoreactive cells were visible at the host-graft interface (Fig. 6). Some immunoreactive cells were also observed in the host retina.
Immunoreactive cells were rare in most parts of transplants obtained at PN 5, 11 and 12 but immunoreactive cells could be seen at the host graft interface [23] and occasionally in the host retina.
Conclusion
The results SHOW that at the time of transplantation the grafts were very disorganized but soon after that they began to organize themselves in the form of rosettes. The arrangement of the cells and retinal layers in the graft is well documented [7,15,28]. Briefly, photoreceptors were present at the luminal side of the rosettes, and were surrounded by layers corresponding to the inner layers of normal retina.
It is interesting to note that the large deeply stained cells in the late phases of cell cycle formed clusters to form the beginning of what would become a rosette. The location of these cells (luminal layers of the rosettes) corresponds to the outer retina where such cells are normally located during the development of the normal retina [24,25].
Randomly distributed postmitotic cells were seen one day after transplantation. They are likely to be ganglion cells which are present already in the E 15 retinas [24,25]. This suggests that E 15 may not be the best time to harvest the donor tissue regarding the survival of the ganglion cells, since it is best to harvest cells before they undergo their terminal mitosis [29,30]. Most transplants have previously been performed with tissue FROM E 15 or older fetuses, and this may partially EXPLAIN why it has hitherto not been possible to see ganglion cells in the transplants. However, later studies have shown that it is easier to obtain well preserved morphology with later stage transplants (see below).
The pattern of proliferation in the retinal transplants resembles the general proliferation pattern seen in normal retinogenesis. The mitotic figures in the normal developing retina accumulate at the outer limiting membrane [24,25]. This results FROM interkinetic migration, in which the proliferating cells synthesize their DNA away FROM the ventricular surface, [24,25], and then migrate to the ventricular surface, to complete mitosis. The results in this study SHOW that in retinal cell transplants, the same pattern is maintained, even though the donor tissue has undergone extensive mechanical handling at the time of transplantation. The luminal surface of the rosettes, corresponds to the ventricular (apical) surface of the normal retina. Cells in metaphase appear in the luminal parts of the rosettes, whereas many other cells in the surrounding layers are in other phases of the mitotic cycle, as indicated by their content of the Ki-67 antigen. The observed picture suggests that there must be interkinetic migration within the rosettes, similar to that seen in the normal retina.
The postmitotic cells in the luminal layers of the rosettes at E 21 and E 22 are likely to be the differentiating photoreceptors, and the proliferating cells in the outer layers of the rosettes are probably giving rise to more photoreceptors and the cells of inner retina The non-proliferating cells in between the rosettes are likely to be the differentiating amacrine and horizontal cells, since these cells are born early in ontogeny [24,25,31,32].
In E 29 and PN 2 transplants the proliferation is mostly confined to the regions in between the rosettes, which correspond to the layers of the inner half of the normal retina. This shift of where proliferating cells predominate is also seen in the normal development of the retina and these proliferating cells are likely to be the Müller cells [24,25].
In the postnatal day 4 transplants and later, only small patches of proliferation were seen. The regional differences in the mitotic activity in the transplant may be due to the mixing of the central and the peripheral parts of the donor retina at the time of transplantation In normal retinas that proliferation ceases in the central retina at birth and a few days later in the peripheral retina [24,25,33].
Four days after the transplantation (in E 19 transplants), numerous dying cells appeared in the parts of the transplant that were closest to the host retina and this degenerating layer was observed in older ages also. The host retina overlying the graft also loses its photoreceptor outer segments and many of its cells in the outer nuclear layer [15,28], suggesting a negative host-graft interaction. The absence of the pyknotic cells on the side of the transplants, and in small transplants suggest that the degeneration of the graft is not related to the proximity to the host retina. It remains to be seen whether it is the lack of nourishment FROM the choroid reaching the distant layers of the graft and the host retina which is responsible for this phenomenon.
Marked proliferation at the host-graft interface may represent abnormal gliosis. In human xenotransplants to rats, cellular retinaldehyde-binding protein (CRALBP) immunoreactivity was found mostly close to the host retina [34] and glial fibrillary acidic protein (GFAP) immunoreactive fibres were also found mostly at the same location. Neural transplants in brain often become encapsulated by a glial barrier which interferes with the integration of the graft with the host [35]. The observations in the present study suggest that the same phenomena may occur in retinal cell transplants also.
The pattern of proliferation in the transplant is comparable to that in normal development, but it seems that the transplanted cells do not proliferate as much as the cells in normal development. For example in E 25 and E 29 normal retinas, the proliferation of cells was much more than at comparable ages in transplants. For comparison, at E 29 the proliferation in the transplant was confined to the areas corresponding to the proliferation in the inner nuclear layer (in between the rosettes). In the normal developing retina, at this stage there is much more proliferation in the inner nuclear layer and also there is proliferation in the outer nuclear layer [24,25]. Further, proliferation in postnatal transplants was minimal, whereas significant proliferation persists up to day PN 7 to PN 11 in normal development. Moreover, cell death in the transplants may also be a important factor retarding the development of the transplants.
Cell proliferation is not the only factor that affect the development of the retina. The growth of the eye and the retina is a complex process involving both active growth and passive stretching [33]. After the retinal cell proliferation ceases the retina passively stretches with the sclera increasing the surface area up to threefold in rabbits at the cost of retinal thickness [33]. It is possible that the forces needed for the passive expansion of the retina are lacking in the case of a retinal graft placed in a developed eye and thus the graft does not undergo the passive stretching and fails to cover the entire surface of the eye.
The proliferating cells in the host retina are likely to be glial cells because Müller cells are known to proliferate after retinal damage [36-38]. Further, there are indications that host Müller cells respond to the transplantation surgery. For instance, they express glial fibrillary acidic protein (GFAP) soon after the surgery, and this reaction spreads out to the whole retina within 1 day. It lasts for at least 7 weeks [18]. Hypertrophy and migration of Müller cells adjacent to the lesion site in the host retina was also noticed [18]. All this is consistent with the proliferating cells being glial cells.
Lamination in retinal cell transplants
One of the problems with the retinal transplant surgery is the formation of rosettes, which means that the retinal layers instead of being organized in flat layers, are organized in the form of spheres which on sectioning (for histological examination) appear like rosettes.
Donor retinas have been transplanted in various physical states. Cell suspensions grafts SHOW very little organization and hardly any photoreceptor outer segments [21,39-43]. Transplanted tissue fragments survive well [28,44-46] and SHOW better lamination than that of suspension technique [21]. The extent of the rosette formation differs with the transplantation technique. With the fragmentation technique, rosettes are a prominent feature [28,44,47], whereas they are much fewer or even absent in cell suspension transplants [21,43].
The pathogenesis of rosette formation is not well understood, but it is thought to be a general injury response. We have therefore modified the tissue fragment procedure so that comparatively large sheets of minimally disturbed fetal retina can be transplanted. Since it requires less mechanical handling of the transplant tissue to place it epiretinally than between the photoreceptors and the pigment epithelium, we have compared the results of transplantation at these two places and obtained by the tissue fragmentation technique. | |
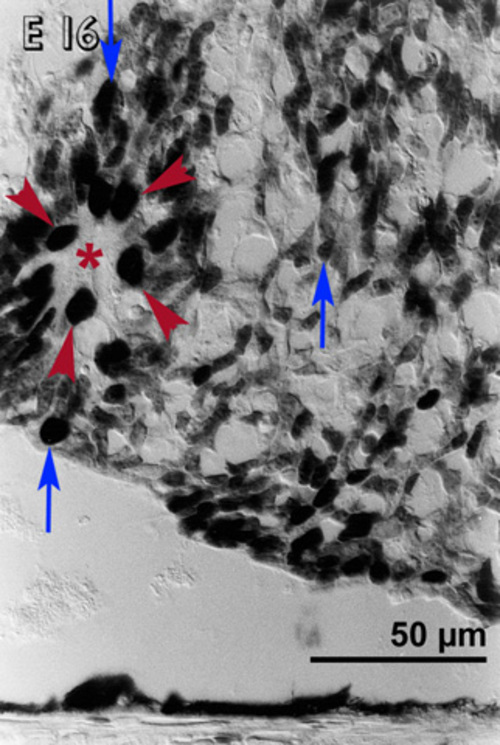
Figure 1
Embryonic day 16 (E 16) rabbit retinal transplant one day after the transplantation. Immunohistochemical staining with the MIB-1 antibody showing homogeneously distributed immunoreactive cells. Certain large deeply staining cells (blue arrows) are scattered throughout the transplant, but at places they are also found (red arrowheads) near the lumen of rosette-like clusters (lumen marked with red asterisks).
|
|
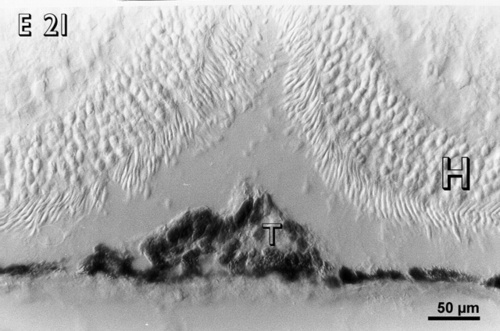
Figure 2
A small embryonic day 21 (E 21) rabbit retinal transplant (T) covered by the host retina (H) shows immunoreactive cells. There are no pyknotic cells in the transplant.
|
|
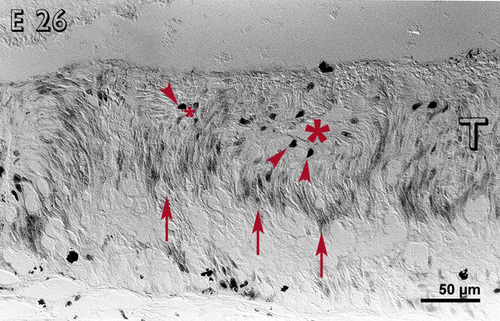
Figure 3
Embryonic day 26 (E 26) rabbit retinal transplant (T) showing rosettes (lumen marked with red asterisks) with deeply stained cells (red arrowheads) towards the outer limiting membrane. The inner layers of the rosettes are non-reactive, whereas the outer layers are reactive (red arrows). Immunohistochemical staining with the MIB-1 antibody.
|
|
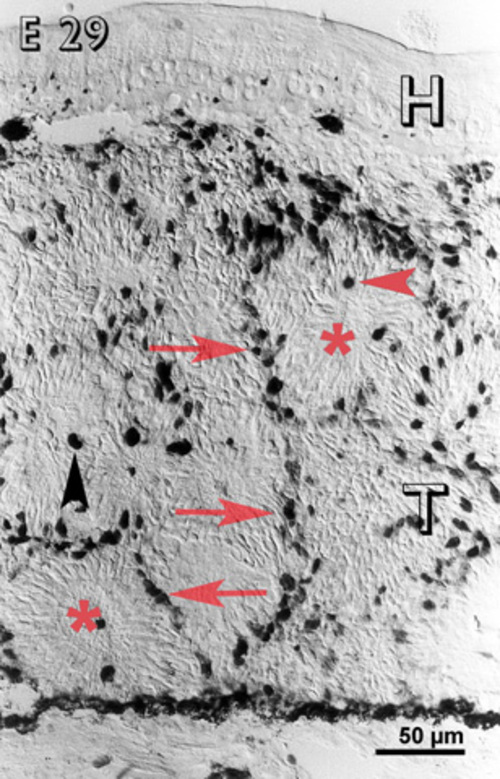
Figure 4
Embryonic day 29 (E 29) rabbit retinal transplant (T) showing reactive cells only in between the rosettes (red arrows) and some deeply stained cells (red arrowhead) towards the lumen of the rosettes (lumina marked with red asterisks). Immunohistochemical staining with the MIB-1 antibody. H = host retina.
|
|

Figure 5
Postnatal day 2 (PN 2) rabbit retinal transplant (T) showing immunoreactive cells in between the rosettes (red arrows) and at the host-graft interface (green arrows). An immunoreactive cell (blue arrow) is visible in the host retina (H). Immunohistochemical staining with MIB-1 antibody.
|
|
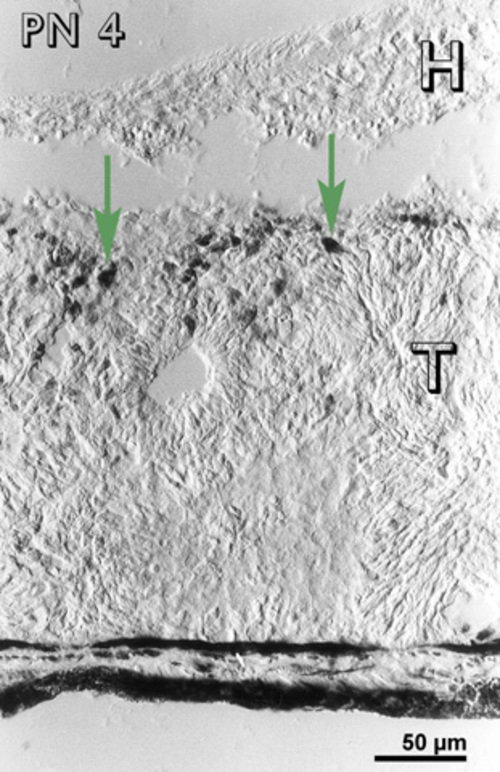
Figure 6
Postnatal day 4 (PN 4) rabbit retinal transplant (T) showing immunoreactive cells at the host-graft interface (green arrows). Immunohistochemical staining with the MIB-1 antibody. H= host retina.
|
|
| Introduction | In 1873, Dooremaal placed cells FROM human labial mucosa and a variety of other tissues in the anterior chamber of rabbit [1], and this was the first published attempt to use the eye as a transplantation site. Shortly afterwards, whole eye transplants were performed, but the results were not encouraging [2]. However, the anterior chamber technique worked and for a long time it served as the method of choice for studying tissue growth in an isolated environment, resulting in a wide variety of tissues being transplanted to this site [3]. Retina was first transplanted to the anterior chamber by Royo and Quay in 1959 [4]. The study demonstrated that contrary to general expectations, transplanted fetal retinal neurons survive, differentiate and develop, but the report went largely unnoticed.
Experimental transplantation of retinal pigment epithelium cells were first reported by Gouras et al. [5]. Lately, patients with macular degeneration and subretinal membranes have received pigment epithelium transplants [6; see also reports by Gouras and Algvere in this Symposium].
The first transplantation of retinal neurons to the posterior segment was done by Turner and Blair [7]. They transplanted neonatal rat retina INTO adult rats, placing it close to a special lesion site. The transplants developed according to their normal timetable but formed so-called rosettes. With different modifications, the same method has been used for allogenic or xenogenic transplants in mice, rats, rabbits, monkey and humans [8-12]. Survival and differentiation of retinal cell transplants have been studied in considerable detail [10,13]. Transplants survive at various transplantation sites [14,15], and they develop all the retinal layers and most of the cell types of the normal retina [9,16-19]. Many of the essential proteins [15,20,21] and the neurotransmitters [16,22] have also been found in the transplants.
With the surgical procedures that have been developed, transplants mature, differentiate and form approximations of the retinal layering, but anomalies like rosettes are always seen. We have now refined our surgical procedure so that we can obtain well laminated transplants in more than half the operations, and this work will be summarized here. Full reports are presented elsewhere [15].
In most retinal cell transplantation, tissue corresponding to at least two retinas is inserted. Nevertheless, the tissue never forms full-sized retinas like they normally would. As a first step in understanding the reasons for this, we have examined the proliferation taking place in retinal cell transplants and compared it with the normal proliferation cycle in the developing retina. The details of our study are reported elsewhere [23].
Cell proliferation in retinal transplants
Like in other neural tissues, the events in the developing retina occur in a precise and predetermined order. However, the first event in the process of development, proliferation, has not been much studied so far. In most procedures so far used, donor tissue FROM several fetal retinas are allowed to survive and to attain postnatal ages, but nevertheless, the size of the resulting transplant always remains limited. Technical difficulties preclude studies of transplant growth done by measuring the size or the volume of the transplants. However, we have in part circumvented this problem by studying the appearance and distribution of a cell proliferation marker. The work gives information on the influence of host environment on the proliferation of grafted cells.
Embryonic donor tissue, depending upon the stage at which it is harvested, contains neuroblastic cells which are still undergoing mitosis at the time of transplantation [24,25]. Suitable markers for proliferating cells have recently become available, and Ki67 is one of them. This antigen, Ki-67, is present in all dividing cells. It has been widely used to study the proliferating cells in different tissues [26,27]. | | | Materials and Methods | Embryos FROM ordinary mixed strain pigmented rabbits FROM stage E 15, E 16 or E 19 were used. Their eyes were enucleated, and the neural retina was dissected away FROM the posterior eyecup under an operating microscope. Care was taken not to damage the neural retina. Close to all of the undamaged fetal neural retina was then obtained in the form of cups which were transplanted to the adult rabbits of the same strain. The transplants were placed in the epiretinal space, both in the epiretinal or the subretinal space, or only in the subretinal space.
Transplantation instrument
An instrument (Fig. 7) was developed to transplant the slightly cupped pieces of embryonic rabbit retinas INTO the adult eyes. The instrument comprises a handle with a thin cannula which serves as a jacket around a thin-walled flat polyethylene tube attached to a precision micro syringe. Donor tissue was sucked INTO the polyethylene tube with the help of the micro syringe. In this process, the slightly cup-shaped neural retina enters the tube as a single piece. There is inevitably some damage to the transplant tissue at the cut margins, but the central portion remains as an intact sheet.
Surgical Technique
Under an operating microscope, the instrument containing the donor tissue was introduced INTO the recipient eye through a pars plana incision and advanced transvitreally until it reached the posterior pole of the eye. Here, the polyethylene tube was pushed out of the cannula, and if required introduced INTO the subretinal space by doing a retinotomy with the same tube that contained the donor tissue. The donor tissue was then injected by pushing the piston of the microsyringe attached to the cannula.
Further improvement of the surgical technique
Adding vitrectomy and an additional retinotomy to the procedure has further refined the surgical technique. This essentially eliminates fluid reflux through the entrance retinotomy when ejecting the transplant and further minimizes the trauma to the donor tissue at the time of transplantation (Ghosh et al. in press 1997).
In brief, a 180º conjunctival flap was raised. A 20 G infusion cannula was sutured to the sclera in the 4 O’clock position 1 mm posterior to the limbus and a balanced salt solution containing adrenaline and heparin was infused. A metal ring for the support of a contact lens was sutured in place with 2 limbal sutures at 4 and 11 o’clock and 2 sclerotomies were made in the 10 and 2 o’clock positions. A vitrectomy contact lens was placed on the cornea. As much vitreous as possible was removed, using a vitreous cutter (Ocutome®, Cooper Medical Devices Corporation, San Leandro, California, U.S.A) and an intraocular illuminator (Ocutome II surgical illuminator® and fibre optic cable).
In ORDER to create a small neuroretinal detachment, a thin flexible polyethylene capillary (0.6 mm and 0.4 mm in outer and inner diameter) was introduced through the sclerotomy at 10 o’clock position. It was attached to a 1.0 ml syringe filled with Ames solution, and supported by a blunt and slightly bent 20 G metal cannula. By penetrating the retina 2-4 mm inferior to the optic nerve, it was positioned in the subretinal space. The fluid FROM the syringe was carefully infused, creating a limited circular retinal detachment with a diameter of approximately 3 mm. A second, smaller retinotomy was made inferior to the first (Figure 8).
The vitrectomy largely eliminated the need a special injection instrument. Instead, the donor tissue could be drawn INTO a slightly conical glass cannula, one end of which was 1.0 mm, and the other 0.6 mm in diameter. The narrow end of the cannula was connected to a 1.0 ml syringe via a polyethylene tube allowing the transplant together with a small amount of the surrounding Ames solution to be drawn in. As the transplant entered the cannula it folded. The cannula was turned, the polyethylene tube and syringe was attached to the wide end, and the transplant was carefully moved towards the narrow end by gently pushing the plunger of the syringe. The cannula was then introduced INTO the eye through the 10 o’clock sclerotomy and INTO the subretinal space by means of the superior retinotomy. As the transplant was pushed out of the cannula and INTO the subretinal bleb, the accompanying fluid passed out INTO the vitreous space through the inferior retinotomy, but the transplant stayed securely in place subretinally, between the 2 retinotomies.
Immunohistochemistry
Standard immunohistochemistry was performed for the following markers: opsin (to demonstrate the photoreceptor outer segment development), vimentin (to demonstrate the overall architecture of the graft by marking the Müller cells), peanut agglutinin (PNA), interphotoreceptor retinoid binding protein (IRBP), glial fibrillary acidic protein (GFAP), HPC-1, choline acetyl transferase (ChAT), neurofilament 160 kDa (NF160), and AB5 (for ganglion cells). | |
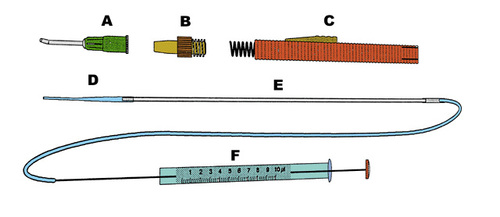
Figure 7
Drawing of the instrument used for transplantation. The plastic tube (D) fits inside the main parts of the instrument (B and C). This part of the instrument pushes the plastic tube (D) out of the cannula (A) which shields it during the passage through the vitreous. The other end of the plastic tube (D) is connected to a glass capillary which in turn is connected to the precision tube (F) that controls the injection of the donor tissue.
|
|
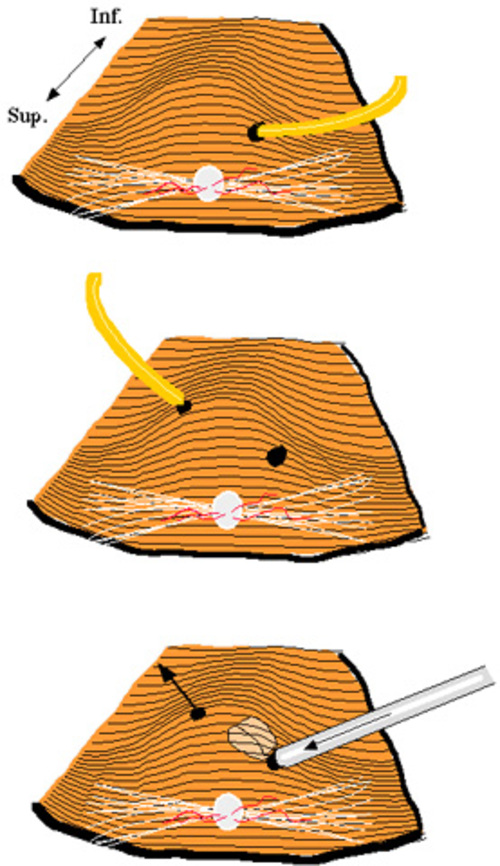
Figure 8
The 3 steps leading to transplantation of the full thickness neuroretina. Surgeon’s view with the optic nerve superiorly. Top: A retinal detachment bleb is created inferior to the optic nerve by penetrating the retina with a polyethylene capillary and infusing Ames’ solution. Middle: A second retinotomy is performed using the polyethylene capillary. Bottom: The transplant has been introduced subretinally by means of the glass cannula. Fluid flows out FROM the second retinotomy, but the transplant stays in place due to its relatively larger size of the donor tissue.
|
|
| Results | It was possible to place flat pieces of whole embryonic retinas in the epiretinal or the subretinal space of the adult rabbits. One of the problems faced during the surgery was that at times the donor retinas came out FROM the subretinal space through the retinotomy. This problem was overcome by making two retinotomies and in addition doing a vitrectomy at the time of transplantation.
In operations performed without vitrectomy, the epiretinal transplants had grown INTO a more or less spherical or cup-shaped laminated sheet like in normal retinogenesis, six weeks after the transplantation (equivalent to 8 weeks post conception). This piece of the donor tissue did not SHOW any rosette formation except at the margin of the transplant [15]. Transplant cells differentiated in two DISTINCT layers. No photoreceptor outer segments were observed on light microscopy (Fig. 9), and their absence was confirmed by an immunohistochemical staining for opsin.
In the subretinal flat transplants in the same eyes, the cells were often arranged in irregular arcuate arrays or rosette-like clusters (Fig. 10) without any DISTINCT center as seen in case of rosettes. The photoreceptors in the rosettes, seen in the transplants made by fragmentation technique as controls, faced their centers. Both in the arrays of cells (new technique) and in the rosettes (fragmentation technique), the photoreceptors most often had well developed outer segments (Fig. 11; opsin staining of the outer segments in arcuate arrays). Particularly in cell arrays, they were also associated with a well developed outer limiting membrane as judged by light microscopy (Fig. 9). The rosettes dominated the tissue fragment transplants.
Both in the arcuate arrays and the rosettes two DISTINCT layer of cell bodies were seen, the first corresponding to the cells of the outer nuclear layer and the other that of the inner nuclear layer. In between these two layers lay the equivalent of the outer plexiform layer. In places, a plexiform layer equivalent to the inner plexiform layer was also seen. The organization was more regular and prominent in the arcuate arrays than in the rosettes. A cell layer resembling the ganglion cell layer was also present., (Fig. 10).
Müller cells contain vimentin, and staining for this substance therefore reveals their overall morphology. As seen in fig. 12, vimentin staining of epiretinal transplants not covered by host retina reveal a Müller cell organization which is indistinguishable FROM that of a normal retina. A similar organization is noticeable in the arcuate arrays (Fig. 13), but not in tissue fragment transplants (Fig. 14).
With the improved technique in which two retinotomies and vitrectomy were performed, the graft attained an even better architecture (Fig. 15).
In most of the transplants with E16 or E19 donor tissue, histology revealed large (up to 1.8 mm) straight, correctly positioned laminated transplants. The animals kept alive up to PN 31 and 35 days displayed all normal retinal layers including photoreceptor outer segments opposed against the host retinal pigment epithelium. Tissue FROM the youngest donors (E15) yielded less well organised transplants indicating a critical age of donor tissue before which transplantation in respect of lamination is less favourable.
In the laminated transplants, immunohistochemistry showed that all the markers for the neurones and the glial cells studied were present. The peanut agglutinin (PNA) immunohistochemistry showed similar labelling as in the host retina. Interphotoreceptor retinoid binding protein (IRBP) immunoreactivity was found in the outer segment region. Normal vimentin stained Müller cells were seen, but glial fibrillary acidic protein (GFAP) immunoreactivity seemed less pronounced than in the host retina. The inner and outer plexiform layers were well outlined by the HPC-1 antibody and this marker also stained the nerve fibre layer. Displaced amacrine cells in the ganglion cell layer were labelled by choline acetyl transferase (ChAT). In the nerve fibre layer, all transplants contained fibres marked by the neurofilament 160 kDa (NF160) antibody. In some transplants the AB5 antibody labelled a few cells in the ganglion cell layer. | |
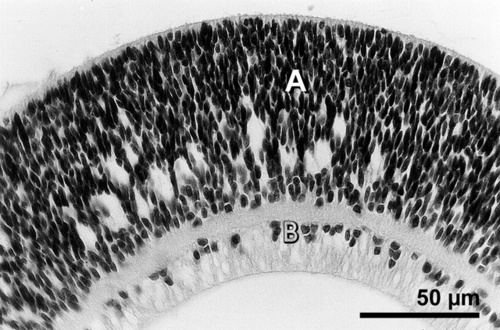
Figure 9
A 6 week old E 15 retinal large sheet transplant in the epiretinal space developing as a spheroid without any rosettes. The transplant shows no photoreceptor outer segments and the graft has differentiated in two nuclear layers. Neuroblastic cell layer (A) is separated FROM the layer that resembles ganglion cell layer (B) by a plexiform layer. Hematoxylin and eosin.
|
|

Figure 10
A 6 week old E 15 large sheet retinal transplant in the same eye as the transplant in Fig. 9, showing the formation of arcuate structures and relatively well developed outer segments with outer limiting membrane (arrow heads). The transplant develops all the retinal layers found in a normal retina, namely the outer nuclear layer (ONL), the inner nuclear layer (INL), and the ganglion cell layer (GCL), and these layers are separated by the plexiform layers. Hematoxylin and eosin H = host retina.
|
|
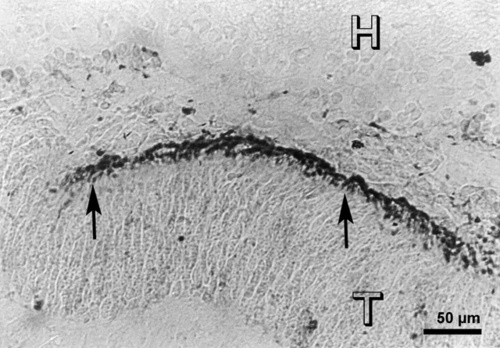
Figure 11
A 6 weeks old E15 large sheet subretinal transplant (T), showing opsin staining in the photoreceptor outer segments (arrows) in arcuate arrays. Immunostaining with opsin antibody R-15, H = host retina.
|
|

Figure 12
Vimentin staining in a large sheet transplant in the epiretinal space. The transplant has become folded because it was not covered by the host retina. It shows a palisade-like arrangement of the Müller cells.
|
|
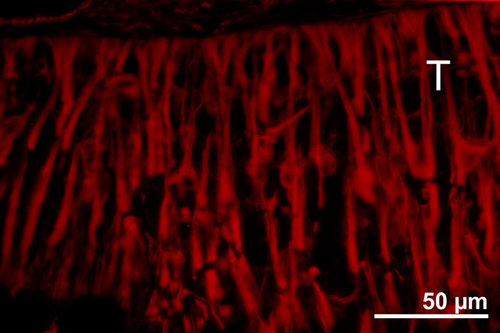
Figure 13
Vimentin staining in a 6 week old E15 large sheet retinal transplant (T) in the subretinal space, showing palisade-like arrangement of Müller cells in arcuate arrays. This arrangement is different FROM the radial arrangement seen in rosettes found in transplants done by the tissue fragment technique like in Fig. 14
|
|
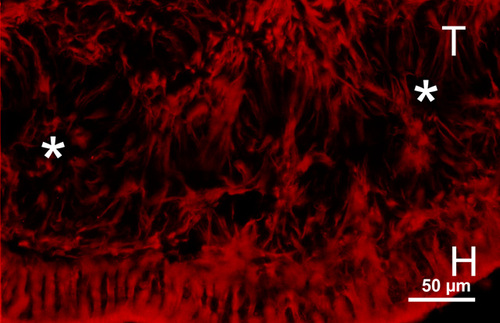
Figure 14
Vimentin staining in a graft (T) transplanted by fragmentation technique in the subretinal space (H = host retina), showing the radial arrangement of Müller cells in the rosettes (white asterisks). This arrangement is different than that of arcuate arrays in the large sheet retinal transplant as shown in Fig. 13.
|
|
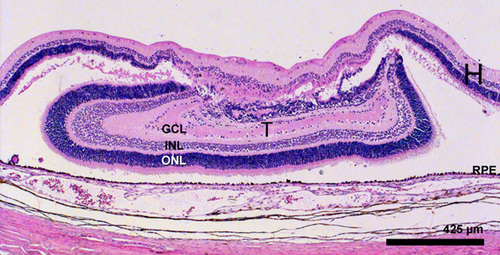
Figure 15
Haematoxylin and eosin staining of a E 19 transplant 35 days postoperatively. The transplant (T) is 1.7 mm in it’s laminated part abutting the host retinal pigment epithelium. Even the edges of the transplant SHOW good lamination. The transplant shows the equvalent of the outer nuclear layer (ONL), the inner nuclear layer (INL), and the ganglion cell layer (GCL) and also two plexiform layers. The host retina (H) is seen to cover the transplant and is degenerating in this area.
|
|
| Discussion | The donor tissue at E 15 consists largely of undifferentiated neuroblastic cells [24,25]. Both in epiretinal and subretinal transplants, the donor tissue was seen to continue to mature and differentiate. Even when the subretinal and the epiretinal transplants were placed in the same eye under identical circumstances, subretinal transplants developed outer segments, whereas the epiretinal ones did not. Further, in subretinal transplants, an outer limiting membrane was found. This suggests that the subretinal transplants matured more rapidly than the epiretinal ones.
Epiretinal transplants were largely free of rosettes except at their margins, where the transplanted retina was traumatized during transplantation, whereas the subretinal transplants formed arcuate structures, and not rosettes
The regeneration of the chicken retina in the early embryonic stages, occurs by two completely different mechanisms in the central and the peripheral parts. The central retina regenerates by transdifferentiation of RPE and produces an inverse pattern of lamination [50-52] whereas regeneration at the ciliary margin results FROM cellular proliferation and establishment of the correct polarity [50,51,53]. Also, in the retinal regeneration models in rotation culture systems, dissociated cells FROM the central retina give rise to inversely laminated rosette spheroids, which is reminiscent of disturbed retinogenesis [54]. Here the initial aggregation process causes clustering of the most adhesive cells. The initial interaction of the embryonic cells is mediated by variety of cell adhesion molecules [53,55,56]. Such molecules also play a role in cellular and histological differentiation [57,58]. Interestingly, the outer segments in these rosettes are facing the center, which is comparable to the reversed orientation of retina regenerated by transdifferentiation of pigmented epithelium in the central retina [50-52]. On the other hand, in the mechanism involved in retinal regeneration FROM the pigmented eye margin resulting in correctly laminated stratospheroids, aggregation of the cells appears to play a minor role. Here, pigmented cells in the cell aggregates induce neuroblasts to proliferate. Factors yet unknown, mediating this process also induce correct laminar orientation in the newly formed cells. Spheroids thus formed do not contain rosettes but laminated spherical structures with correct polarity, that is the photoreceptors face the outside, similar to the in vivo regeneration FROM the peripheral retina.
Our transplants are comparable to the regeneration model of central retina, where the tissue develops by a continuation of the differentiation rather than by proliferation. The fragment technique gives rise to rosettes with the photoreceptors facing their centers like in regeneration of the central retina both in vivo and in vitro.
The results with epiretinal transplants with our new technique SHOW that the initial reaggregation of the cells is important for the eventual cytoarchitecture of the transplant. When the structural disturbance of the donor tissue is minimal at the time of transplantation, the eventual structure has few or no rosettes. The results also suggest that the dissection and handling of the fetal tissue to be transplanted can be performed carefully enough to prevent rosette formation.
With the new improved procedure involving two retinotomies and the vitrectomy procedure, it was possible to transplant embryonic retina to the appropriate position adjacent to the host retinal pigment epithelium, keeping the transplant architecture intact. The transplants SHOW good layering and well developed photoreceptors abutting the retinal pigment epithelium. Immunohistochemistry demonstrated that almost all the markers studied were present in the laminated grafts. PNA was previously found to be abnormal, but was now found to be normal in the well laminated transplants. Transplants made with the tissue fragment technique lacked IRBP whereas this could be demonstrated in the laminated transplants [20]. Further, no AB5 immunoreactive cells were seen in transplants obtained with the fragment technique transplants but were found in the well laminated transplants. This suggests that the architectural organisation of the transplant is important for proper development.
In brief, it is important to maintain the tissue architecture at the time of dissection and transplantation in ORDER to get a desirable lamination. In addition, the differences in the degree of photoreceptor development suggest that the site of transplantation influenced the development of the transplants. | | | Acknowledgements | | This work was supported by the Swedish Medical Research Council (project 14X-2321), the RP Foundation Fighting Blindness, the Segerfalks stiftelse, the H och L Nilssons stiftelse, Kronprinsessan Margaretas Arbetsnämnd för synskadade, Stiftelsen Clas Groschinskys Minnesfond, Riksbankens Jubileumsfond, the Swedish Society for Medical Research, the EU BioMed 2 program, and the Medical Faculty of the University of Lund. | | | References | 1. van Dooremaal JC, De ontwikkeling van levende veefsels op vreemden boden geent. Onderz physiol Lab Rijksuniv. Utrechsche Loogeschool 3, 277, (1873)
2. May, CH, Transplantation of a rabbits eye INTO the human orbit. Arch.Ophthalmol. 16, 47, (1887)
3. Olson, L, Björklund, H, and Hoffer, BJ, Neural transplants. Development and function" (J. R. Sladek,Jr. and D. M. Gash, Eds.), pp. 125-165. Plenum Press, New York, London, (1984)
4. Royo, PE and Quay, WB, Retinal transplantation FROM fetal to maternal mammalian eye. Growth 23, 313, (1959)
5. Gouras, P, Flood, MT, Eggers, HM, and Kjeldbye, HM, Invest.Ophthalmol.Vis.Sci. 24, ARVO suppl, 142, (1983)(Abstract)
6. Algvere, P, Berglin, L, Gouras, P, and Sheng, Y, Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularisation. Graefe's Arch.Clin.Exp.Ophthalmol. 232, 707, (1994)
7. Turner, JE and Blair, JR, Newborn rat retinal cells transplanted INTO a retinal lesion site in adult host eyes. Brain Res.(Dev.Brain Res.) 391(26), 91, (1986)
8. Aramant, R and Turner, JE, Cross-species grafting of embryonic mouse and grafting of older postnatal rat retinas INTO the lesioned adult rat eye: the importance of cyclosporin A for survival. Brain Res.(Dev.Brain Res.) 469(41), 303, (1988)
9. Ehinger, B, Bergström, A, Seiler, M, Aramant, RB, Zucker, CL, Gustavii, B, and Adolph, AR, Ultrastructure of human retinal cell transplants with long survival times in rats. Exp.Eye Res. 53, 447, (1991)
10. Sharma, RK, Bergström, A, and Ehinger, B, Retinal cell transplants. Prog.Retinal Eye Res. 15, 197, (1995)
11. Sharma, RK and Ehinger, B, Retinal transplants; how close to clinical application? Acta Ophthalmol. Scandinevia 75, 355, (1997)
12. Ehinger, B, Juliusson, B, Bergström, A, and Sharma, RK, Photoreceptor development in retinal cell transplants. In: Kato S, Osborne NN & Tamai M (eds). 155, Kugler publications. (1996)
13. Sharma, RK, Development of retinal transplants (Dessertation) University of Lund, Sweden (1996)
14. Bergström, A, Embryonic rabbit retinal transplants survive and differentiate in the choroid. Exp.Eye Res. 59, 281, (1994)
15. Sharma, RK, Bergström, A, and Ehinger, B, Influence of technique and transplantation site on rosette formation in rabbit retinal transplants. Acta Ophthalmol. Scandinevia 75, 3, (1997)
16. Bergström, A, Ehinger, B, Wilke, K, Zucker, CL, Adolph, AR, and Szél, ×, Development of cell markers in subretinal rabbit retinal transplants. Exp.Eye Res. 58, 301, (1994)
17. Ehinger, B, Zucker, C, Bergström, A, Seiler, M, Aramant, R, and Adolph, A, Electron microscopy of human first trimester and rat mid-term retinal cell transplants with long development time. Neuro-ophthalmology 12, 103, (1992)
18. Seiler, M and Turner, JE, The activities of host and graft glial cells following retinal transplantation INTO the lesioned adult rat eye: developmental expression of glial markers. Brain Res.(Dev.Brain Res.) 471(43), 111, (1988)
19. Sharma, RK, Perez, MTR, and Ehinger, B, Immunocytochemical localization of nitric oxide synthase in developing and transplanted rabbit retinas. Histochem. Cell Biol. 107, 449, (1997)
20. Juliusson, B, Mieziewska, K, Bergström, A, Wilke, K, van Veen, T, and Ehinger, B, Exp.Eye Res. (1994)(in press)
21. Juliusson, B, Bergström, A, van Veen, T, and Ehinger, B, Cellular organization in retinal transplants using cell suspensions or fragments of embryonic retinal tissue. Cell Transplant. 2, 411, (1993)
22. Aramant, R, Seiler, M, Ehinger, B, Bergström, A, Adolph, AR, and Turner, JE, Neuronal markers in rat retinal grafts. Brain Res.Dev.Brain Res. 53, 47, (1990)
23. Sharma, RK and Ehinger, B, Cell proliferation in retinal transplants. Cell Transplant. 6, 141, (1997)
24. Sharma, RK, Juliusson, B, and Ehinger, B, Invest.Ophthalmol.Vis.Sci. 37, S692, (1996)(Abstract)
25. Sharma, RK and Ehinger, B, Mitosis in developing rabbit retina: an immunohistochemical study. Exp.Eye Res. 64, 97, (1997)
26. Bilous, AM, McKay, M, and Milliken, J, A comparison between Ki-67 antibody reactivity and other pathological variables in breast carcinoma. Pathology. 23, 282, (1991)
27. Houmand, A, Abrahamsen, B, and Tinggaard Pedersen, N, Relevance of Ki-67 expression in the classification of non-Hodgkin's lymphomas: a morphometric and double-immunostaining study. Histopathology 20, 13, (1992)
28. Bergström, A, Ehinger, B, Wilke, K, Zucker, CL, Adolph, AR, Aramant, R, and Seiler, M, Transplantation of embryonic retina to the subretinal space in rabbits. Exp.Eye Res. 55, 29, (1992)
29. Brundin, P, Barbin, G, Strecker, RE, Isacson, O, Prochiantz, A, and Björklund, A, Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro. Brain Res. 467, 233, (1988)
30. Brundin, P, Nilsson, OG, Strecker, RE, Lindvall, O, Åstedt, B, and Björklund, A, Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson's disease. Exp.Brain Res. 65, 235, (1986)
31. Reichenbach, A, Kasper, M, Schnitzer, J, Osborne, NN, and Pritz Hohmeier, S, A set of early-born neurons is distinctly labeled by several defined antibodies in the adult rabbit retina. J.Hirnforsch. 35, 391, (1994)
32. Reichenbach, A and Robinson, SR, Phylogenetic Constraints on Retinal Organisation and Development. Prog.Retinal Eye Res. 15, 139, (1995)
33. Reichenbach, A, Schnitzer, J, Friedrich, A, Ziegert, W, Brückner, G, and Schober, W, Development of the rabbit retina. I. Size of eye and retina, and postnatal cell proliferation. Anat.Embryol.(Berl) 183, 287, (1991)
34. Seiler, MJ and Aramant, RB, Photoreceptor and glial markers in human embryonic retina and in human embryonic retinal transplants to rat retina. Dev.Brain Res. 80, 81, (1994)
35. Azmitia, EC and Whitaker, PM, Formation of a glial scar following microinjection of fetal neurons INTO the hippocampus or midbrain of the adult rat: an immunocytochemical study. Neurosci.Lett. 38, 145, (1983)
36. Inomata, H, Wound healing after xenon arc photocoagulation in the rabbit retina. Identification of the proliferating cells in the lesion by light and electron microscopic autoradiography using 3h-tymidine. Ophthalmologica 170, 462, (1975)
37. Laqua, H and Machemer, R, Glial cell proliferation in retinal detachment (massive periretinal proliferation). Am.J.Ophthalmol. 80, 602, (1975)
38. Nork, TM, Ghobrial, MW, Peyman, GA, and Tso, MOM, Massive retinal gliosis. A reactive proliferation of Müller cells. Arch.Ophthalmol. 104, 1383, (1986)
39. del Cerro, M, Notter, MF, Wiegand, SJ, Jiang, LQ, and del Cerro, C, Intraretinal transplantation of fluorescently labeled retinal cell suspensions. Neurosci.Lett. 92, 21, (1988)
40. del Cerro, M, Notter, MFD, del Cerro, C, Wiegand, SJ, Grover, DA, and Lazar, E, Intraretinal transplantation for rod-cell replacement in light-damaged retinas. J.Neural Transplant. 1, 1, (1989)
41. Gouras, P, Du, J, Gelanze, M, Kwun, R, Kjeldbye, H, and Lopez, R, Transplantation of photoreceptors labeled with tritiated thymidine INTO RCS rats. Invest.Ophthalmol.Vis.Sci. 32, 1704, (1991)
42. Gouras, P, Du, J, Kjeldbye, H, Kwun, R, Lopez, R, and Zack, DJ, Transplanted photoreceptors identified in dystrophic mouse retina by a transgenic reporter gene. Invest.Ophthalmol.Vis.Sci. 32, 3167, (1991)
43. Gouras, P, Du, J, Kjeldbye, H, Yamamoto, S, and Zack, DJ, Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest.Ophthalmol.Vis.Sci. 33, 2579, (1992)
44. del Cerro, M, Gash, DM, Rao, GN, Notter, MF, Wiegand, SJ, and Gupta, M, Intraocular retinal transplants. Invest.Ophthalmol.Vis.Sci. 26, 1182, (1985)
45. Blair, JR and Turner, JE, Optimum conditions for successful transplantation of immature rat retina to the lesioned adult retina. Brain Res.(Dev.Brain Res.) 433(36), 257, (1987)
46. Seiler, M, Aramant, R, Ehinger, B, and Adolph, AR, Soc.Neurosci.Abstr. 14, Part 2, 1276, (1988)(Abstract)
47. McLoon, LK, Lund, RD, and McLoon, SC, Transplantation of reaggregates of embryonic neural retinae to neonatal rat brain: differentiation and formation of connections. J.Comp.Neurol. 205, 179, (1982)
48. Lahav, M, Albert, DM, and Craft, JL, Light and electron microscopic study of dysplastic rosette-like structures occurring in the disorganized mature retina. Albrecht v.Graefes Arch.klin.exp.Ophthal. 195, 57, (1975)
49. Milam, AH and Jacobson, SG, Photoreceptor rosettes with blue cone opsin immunoreactivity in retinitis pigmentosa. Ophthalmology 97, 1620, (1990)
50. Guérin, CJ, Anderson, DH, Fariss, RN, and Fisher, SK, Retinal reattachment of the primate macula. Photoreceptor recovery after short-term detachment. Invest.Ophthalmol.Vis.Sci. 30, 1708, (1989)
51. Coulombre, JL and Coulombre, AJ, Regeneration of neural retina FROM the pigmented epithelium in the chick embryo. Dev.Biol. 12, 79, (1965)
52. Coulombre, JL and Coulombre, AJ, Influence of mouse neural retina on regeneration of chick neural retina FROM chick embryonic pigmented epithelium. Nature 228, 559, (1970)
53. Linnemann, D and Bock, E, Cell adhesion molecules in neural development. Dev.Neurosci. 11, 149, (1989)
54. Layer, PG and Willbold, E, Embryonic chicken retinal cells can regenerate all cell layers in vitro, but ciliary pigmented cells induce their correct polarity. Cell Tissue Res. 258, 233, (1989)
55. Edelman, GM, Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu.Rev.Neurosci. 7, 339, (1984)
56. Rathjen, FG, Neural cell contact and axonal growth. Curr.Opin.Cell Biol. 3, 992, (1991)
57. Takeichi, M, Inuzuka, H, Shimamura, K, Fujimori, T, and Nagafuchi, A, Cadherin subclasses: differential expression and their roles in neural morphogenesis. Cold Spring Harbor Symp.Quant.Biol. LV, 319, (1990)
58. Takeichi, M, Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451, (1991) | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in