|
|
 |
 |
 |
 |
|
|
Can a Glaucoma Shunt Tube Be Safely Extended to the Lacrimal Sac or the Ethmoid Sinus in Keratoprosthesis Patients?
Digital Journal of Ophthalmology 2001
Volume 7, Number 3
May 1, 2001
|
Printer Friendly
|



Claes H Dohlman MD PhD | Massachusetts Eye and Ear Infirmary, Schepens Eye Research Institute and Harvard Medical School, Boston, MA Cynthia Grosskreutz MD PhD Eric J Dudenhoefer MD Peter A D Rubin MD Institutional Affiliation | MEEI, Harvard Medical School-
|
|
|
| Abstract | Objective
Keratoprosthesis in cicatrizing diseases (chemical burns, ocular cicatricial pemphigoid, Stevens-Johnson Syndrome, etc) is often complicated by severe glaucoma. A glaucoma shunt is often valuable but the development of an unusually thick capsule around the shunt plate can still leave the pressure unacceptably high. Theoretically, the intraocular pressure may then be lowered by connecting additional tubing FROM the shunt plate to a cavity with an epithelial lining where fibrous tissue may not form to block the aqueous flow, i.e. the lacrimal sac or the sinuses. In exploring this hypothesis, it must be shown that immediate intraocular infection is not a major problem.
Methods
Three patients with corneal blindness due to Stevens-Johnson Syndrome, repeated graft failures, and ocular cicatricial pemphigoid, respectively, had keratoprosthesis and Ahmed glaucoma valve implanted. Silicone tubing was placed between the shunt plate and the lacrimal sac (one case) or the ethmoid sinuses (two cases). The patients were followed for six, five and two months respectively.
Results
No infection occurred in any of the patients and the intraocular pressures remained normal.
Conclusion
On a short term basis it seems that implanting tubing to divert aqueous humor FROM a glaucoma shunt (distal to the valve) to the lacrimal sac or ethmoid sinuses will not necessarily result in ocular infection in keratoprosthesis patients. This paves the way for long term studies on the intraocular pressure -lowering effect of such shunts.
Keywords
glaucoma shunt, keratoprosthesis | | | Introduction | In severe glaucoma, usually after one or more failed trabeculectomies, a glaucoma tube shunt may have to be implanted.. Some designs include a valve mechanism. Although usually successful in reducing the intraocular pressure initially, a fibrous capsule can form around the shunt plate within a month or two(1).This capsule causes increased resistance to the outflow of aqueous, thus raising the intraocular pressure to sometimes unacceptable levels.
After implanting a keratoprosthesis (KPro) INTO the cornea in cases of severe disease where a standard graft is deemed hopeless, glaucoma is a frequent and troublesome complication.(2) Despite the simultaneous implantation of one or more shunt plates, and supplementation with antiglaucomatous medication, the intraocular pressure may remain insufficiently reduced. This occurs especially in cicatrizing conditions such as after chemical burns, ocular cicatricial pemphigoid (OCP) or Stevens-Johnson syndrome (SJS). The formation of a very thick capsule around the glaucoma device plate constitutes the main obstacle to postoperative aqueous flow in such cases.
Recognition of this problem raises the question: can the shunt tube be safely extended to a site where no fibrous capsule would be expected to form, such as in a cavity with an epithelial lining? Thus, if a tube could be made to extend FROM the shunt plate, distal to the valve, and end in the lacrimal sac or in any of the sinuses, fibrous tissue may not block the flow of aqueous and the pressure might remain at the level of the opening pressure of the valve. In ORDER to test this hypothesis we felt that it must be shown not only that the intraocular pressure remain within the desired range for a long time but also that rapid retrograde infection not be instigated in these keratoprosthesis patients that are notoriously vulnerable to endophthalmitis(3).
A review of the literature reveals earlier attempts to divert aqueous to distant sites. Thus shunts have been connected to the superficial temporal vein(4) or a vortex vein(5). One early report FROM India describes placing a tube (non-valved) INTO the lacrimal sac in aphakic patients, without resulting in infection(6). A valved tube shunt FROM the anterior chamber to the external ocular surface in monkeys has been described(7). In a more recent study on dogs, a non-valved tube was extended to the frontal sinus in four animals. They were followed for up to 18 weeks, again without evidence of infection(8). | | | Materials and Methods | Institutional Review Board approval was obtained for this protocol. All patients were counseled regarding the risks, benefits and alternatives of this procedure, and signed informed consent.
The glaucoma shunt used in these patients was the standard Ahmed valve shunt, model S-2 (New World Medical, Inc., Rancho Cucamonga, CA). The connecting tube, supplied by the same company, was a silicone tube of 6-8 cm. length (outer diameter 0.635 mm, inner diameter 0.305 mm), which was attached to a Tube Extender, model TE, by 10-0 nylon sutures (Fig).
The KPro and glaucoma shunt surgery was performed, as has been described earlier(9). The intraocular pressure was estimated by finger palpation since the rigidity of the devices precluded the use of standard tonometers(2). | |
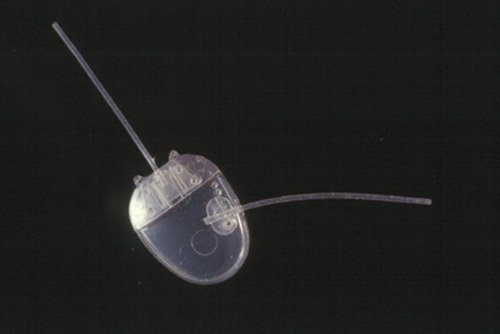
Figure
An Ahmed Tube Extender sutured to an Ahmed valve shunt plate. A similar arrangement was used in the three cases but the extension tube lengths varied.
|
|
| Results | Case 1: The patient is a 52-year old woman of Afro-American descent who suffered a severe attack of SJS eighteen months earlier. She underwent three penetrating keratoplasties, which melted and failed. During this time she was also diagnosed with Systemic Lupus Erythematosus. She was undergoing treatment with Cytoxan (750 mg every four weeks), Dapsone ( 100 mg QD), Doxycycline ( 100 mg. BID) and Prednisone (15 mg./day). Dry eyes and ocular surface inflammation had reduced vision to hand motion with poor projection OU. The intraocular pressures were normal.
The surgery involved implantation of a KPro (Dohlman-Doane Type II) within a fresh corneal graft - a "through-the-lid" arrangement. Because of the virtual certainty of subsequent glaucoma problems after KPro in this SJS patient, an Ahmed single plated valve shunt (model S-2) was placed in the upper nasal quadrant. A 6 mm. piece of silicone tubing had one end slit open slightly and was sutured to the plate (2 holes drilled) with 6-0 Prolene sutures, similar to the arrangement shown in the Figure. It was primed with saline. The lacrimal sac was approached with a standard external dacryocystorhinostomy incision. The upper portion of the sac was exposed and incised. Lacrimal sac irrigation confirmed patency of the nasolacrimal duct. The end of the silicone tubing was lead through the soft tissues, with some slack, and was inserted INTO the sac without anchoring sutures. The incisions were then closed.
The postoperative period was uneventful aside FROM a minor skin adjustment around the KPro nub. A daily dose of 500 mg. Biaxin was given for two months and the immunosuppressive medication was continued without change. Ofloxacin 0.3%, vancomycin (14mg/ml) and medroxyprogesterone (1% suspension) were dropped on the skin area around the plastic nub, four and later three times daily. The intraocular pressure was found to be less than 20 throughout, estimated by palpation. Vision reached the 20/20 level within a month postoperatively.
Case 2: The patient is an 82-year old Caucasian man. On referral in January of 2001, the right eye had light perception without projection. There was a history of amblyopia, angle closure glaucoma, central retinal vein occlusion, intraocular hemorrhage and iris rubeosis. The left eye had a history of angle closure glaucoma, cataract extraction with inflammatory complications, three failed grafts and two Ahmed glaucoma shunts (upper nasal and temporal placements). There was also a history of peripheral retinal detachment, visible with ultrasound. The intraocular pressure was over 30 mm. Hg. in OD, about 40 mm. in OS, while on Cosopt Bid and Alphagan Bid OU.
A KPro Type I in a fresh graft was implanted, plus removal of the IOL and vitrectomy in the OS. Silicone tubing was directed FROM the nasal shunt plate to the anterior ethmoid sinuses adjacent to the posterior lacrimal sac fossa. Thus, an Ahmed tube extender, sutured to the tubing, was inserted INTO the space between the capsule and the shunt plate and sutured to the capsule with 6-0 Prolene. After exposing the posterior lacrimal sac fossa a small osteotomy was made and a large anterior ethmoid air cell was identified. The tubing was then drawn through the subcutaneous tissue to the ethmoid area. The distal tip of the tube was carefully positioned within the air cell not to abut the walls of the sinus, and anchored to the periosteum. The postoperative course was uneventful. Systemic antibiotics were given only for a week but topical medication was the same as in case one. The intraocular pressure rose FROM approximately 5 to 10 mm. Hg. by palpation and has remained at that level. Visual acuity improved only to 20/400 due to previously unrecognized severe optic atrophy with a very pale disc.
Case 3: A 77-year old Caucasian woman with end-stage OCP. Vision was hand movements OU because of dry, inflamed ocular surfaces and repeated ulcerations. Systemic treatment was Cytoxan 750 mg. IV once monthly and prednisone 20 mg/day, which was later reduced to 5 mg/day. In June of 2001 she had a KPro Type II in a corneal graft, removal of IOL, vitrectomy, Ahmed shunt and tarsorrhaphy in the OS. Within two months the intraocular pressure had risen to at least 30 mm. Hg. despite the presence of the shunt. Therefore, silicone extension tubing was placed between the shunt and the ethmoid sinuses with a technique identical to that in case two.
The postoperative course was uneventful. At the one month interval follow up vision had improved to 20/80 and the pressure was approximately10 mm. Hg. by palpation. After two months vision had increased to 20/40 and the pressure was about 20 mm. Hg. | | | Discussion | Previous attempts at diverting aqueous to a distant site have not been popularized because of problems with the device, regurgitation of blood FROM veins, uveitis and other factors. However, it is highly significant that none of the investigators have reported any infection. The purpose of this communication is to SHOW the lack of infection in the immediate postoperative period after keratoprosthesis surgery in these three cases (Table). Retrograde bacterial invasion FROM the nose INTO the eye is certainly a theoretical possibility in this setting, even through the flow of aqueous through the tube as well as the narrow valve opening would be expected to counter such migration.
It is reassuring that no endophthalmitis has occurred so far, given that patients with SJS and OCP are particularly vulnerable to infection after KPro 3. Such patients receive prophylactic antibiotics (vancomycin and ofloxacin) drops on an indefinite basis to the KPro area. This regimen seems to be effective in preventing surface colonization with gram-positive bacteria and subsequent invasion INTO the eye along the surfaces of the plastic device. However, it is unlikely that this surface treatment is capable of preventing an infection FROM the nose through the shunt tube INTO the eye. Patients with chronic sinus infection might not be suitable candidates for this procedure. If endophthalmitis were to occur in the future in eyes with a KPro, Ahmed shunt, and extension tube to the nasal cavities, it would be difficult to tell with certainty by which route the microbes had entered the eye - FROM the nose or FROM the skin surface around the nub of the KPro.
Evaluation of sustained pressure lowering effect FROM the connecting tube will require years of follow up. Although no complications have yet occurred in our three cases, epithelial ingrowth is a potential long-term problem with this procedure. New models of the tube that allow better anchoring to the tissue are presently being developed. Simplification of the surgery is also certainly desirable. Based on this experience, however, it seems reasonable to continue this development in view of the initial absence of infection. | | | References | 1. Melamed C, Cahane M, Gutman R, Blumenthal M: Post-operative complications after
Molteno implant surgery. Am J Ophthalmol 1991;111:319-322.
2. Netland PA, Terada H, Dohlman CH: Glaucoma associated with keratoprosthesis.
Ophthalmology 1998 Apr; 105:751-757.
3. Nouri M, Terada H, Alfonso EC, Foster CS, Durand ML, Dohlman CH: Endophthalmitis
after keratoprosthesis: incidence, bacterial causes and risk factors. Arch Ophthalmol 2001
Apr;119:484-489
4. Rayah-Sivayoham ISS: Camero-venous shunt for secondary glaucoma following orbital
venous obstruction. Br J Ophthalmol 1968; 52:843
5. Lee P, Ward RH: Aqueous-venous shunt for glaucoma. A further report. Arch Ophthalmol
1981; 99:2007-12
6. Mascati NT: A new surgical approach for the control of a class of glaucomas. Int Surg 1967;
47:10
7. Camras CB et al: Valved tube shunt FROM the anterior chamber to the external ocular surface
for use in refractory glaucoma. Invest Ophthalmol Vis Sci 33 (Suppl) 1992; 949
8. Cullen CL, Allen AL, Grahn BH: Anterior chamber to frontal sinus shunt for the division
of aqueous humor: a pilot study in four normal dogs. Vet Ophthalmol 1998;1:31-39.
9. Dohlman CH, Waller S, Netland P: Keratoprosthesis In: Lindquist T and Lindstrom R (eds)
Ophthalmic Surgery UPDATE #4, Mosby-Year Book, Inc. Chicago, V-2-0-24, 1996. | | | Tables |
Diagnosis |
Follow-up |
Vision |
IOP |
Comments |
SJS |
6 mos. |
20/20 |
~ 15 |
clear media |
End stage glaucoma / graft failures |
5 mos. |
20/400 |
~ 10 |
optic atrophy |
OCP |
2 mos. |
20/40 |
~ 20 |
clear media |
Table. Summary of follow-up time and intraocular pressure (estimated by palpation) of the three cases. No infection has occurred to date. The patients are not receiving any glaucoma medication. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in