|
|
 |
 |
 |
 |
|
|
Povidone-iodine concentration and in vitro killing time of bacterial corneal ulcer isolates
Digital Journal of Ophthalmology 2018
Volume 24, Number 4
December 19, 2018
DOI: 10.5693/djo.01.2018.06.001
|
Printer Friendly
Download PDF |


 Jagger C. Koerner, MD
Jagger C. Koerner, MD | Department of Ophthalmology, Albany Medical College, Albany, New York Mary J. George, PhD | Department of Microbiology, Albany Medical College, Albany, New York Elizabeth A. Kissam, BS | Department of Microbiology, Albany Medical College, Albany, New York Michael G. Rosco, BS | Albany Medical College, Albany, New York
|
|
|
| Abstract | Background
The concentration and dosing of povidone-iodine (PI) solution used in surgical site prophylaxis are variable. Prior in vitro work has demonstrated that dilute PI solutions (<1%) had greater bactericidal activity than stock solutions (10%). Studies using pathologic clinical isolates from the eye have yielded mixed results. The purpose of the current study was to evaluate the efficacy of different concentrations of PI on pathologic ocular surface isolates.
Methods
We conducted an in vitro microbiology study using clinical isolates from corneal ulcers. Bacteria were recovered from trypticase soy agar with 5% sheep erythrocytes, chocolate agar, and thioglycollate broth media. A standardized concentration of each bacterial sample (1 × 108 cfu/ml) was exposed to various dilutions of PI. Quantitative cultures were performed to determine the number of organisms surviving PI exposure.
Results
None of the isolates survived exposure to the PI 0.25% solution for 30 seconds. Micrococcus luteus and Staphylococcus aureus survived both 30-second and 1-minute exposure to PI 5% and 10%. The exposure time required to produce no growth was variable with concentrations of <0.25%. In some isolates, the 10% solution was faster than the more dilute solutions (0.1%, 0.05%).
Conclusions
Our results are consistent with prior in vitro studies of PI, from nonocular sources, and suggest that PI has similar bactericidal action on pathologic bacteria from the ocular surface. In vitro exposure to dilute PI (0.25%) resulted in no growth after 30 seconds, whereas 10% and 5% solutions took longer to kill several of the isolates. Future investigations of PI use in ophthalmology as an antimicrobial agent should include the study of low-concentration PI (0.25%).
| | | Introduction | Iodine has been recognized as an effective bactericide since the 1800s. Clinically, iodine is used as a complex of the polymer polyvinylpyrrolidone and iodine, or povidone-iodine (PI). The concentration and dosing of PI used in surgical site prophylaxis are variable. When povidone (a dextran-like polymer) and iodine are mixed in solution, a chemical reaction creates mostly povidone-iodine complex, with a small amount of free iodine. The PI 10% commercial formulation consists of 90% water, 8.5% povidone, and 1% available iodine and iodide.(1) Exposure to organic substances reduces PI’s bactericidal activity by complexing the iodine and by reducing it to iodide.(2) Free iodine is the bactericidal component of PI solution. The concentration of free iodine in solution is concentration dependent and peaks around 0.1%-1%.(1) In 1982 Berkelman et al demonstrated in vitro that dilute PI solutions (<1%) had greater bactericidal activity than stock (10%) solutions. The increased availability of bactericidal free iodine in dilute PI is thought to be a result of weaker iodine linkage to the carrier polymer (povidone) in these solutions.(3,4) Additional in vitro studies have supported this, while those using pathologic clinical isolates from the eye have yielded mixed results.(5,6)
PI is an attractive antimicrobial compound for many reasons, including low cost, universal availability, lack of inducible resistance, and wide spectrum of activity, which includes bacteria, fungi, and viruses.(1) Isenberg et al recently reported using PI 1.25% as a treatment for bacterial keratitis.(7) The efficacy of variable concentrations of PI on pathologic ocular surface isolates has not, to our knowledge, been reported previously, despite the fact that concentration and frequency of use in treatment studies could affect outcomes and the perceived viability of PI in applications beyond surgical site prophylaxis. The purpose of the current study was to evaluate the killing time of various concentrations of PI using clinical isolates from corneal ulcers.
| | | Materials and Methods | | This study is an in vitro microbiology study using clinical isolates from corneal ulcers. A PI 10% solution was used as the stock solution. This was used to make various dilutions using sterile deionized water. Bacteria were recovered from trypticase soy agar with 5% sheep erythrocytes, chocolate agar and thioglycollate broth media that was incubated at 35o C. Plated media were incubated for 48 hours and liquid media for 5 days. Full genus and species identification and susceptibility testing was performed. Recovered bacteria were stored frozen at −70o C until challenge testing. A standardized concentration of each bacterial sample (1 × 108 colony-forming units per ml) was exposed to various dilutions of PI. After the exposure time elapsed, the remaining iodine was neutralized with sodium thiosulfate 0.5%. Quantitative cultures were performed to determine the number of organisms surviving after PI exposure. Nine concentrations of PI solution (0.01%, 0.05%, 0.1%, 0.25%, 0.5%, 1%, 2.5%, 5%, 10%) were tested at exposure times of 15 seconds, 30 seconds, 1 minute, and 5 minutes. | | | Results | | Of the 9 isolates, 6 were killed within 15 seconds by a wide range of PI concentrations (5%-0.05%): Moraxella nonliquefaciens, Probionibacterium acnes, Staphylococcus epidermidis, Streptococcus pneumoniae, Klebsiella oxytoca, and Pseudomonas aeruginosa (Table 1). The M. luteus and S. aureus did not survive exposure to PI 0.25% for 30 seconds, whereas the 5% and 10% solutions produced no growth at the 5 minute exposure time in these isolates. The time required to produce no growth was variable with concentrations of <0.25%. In some cases, the 10% solution was faster; in other cases, the more dilute solutions (0.1%, 0.05%) were faster. The 0.01% solution did not produce a faster killing time than the 10% solution in any of the isolates. No isolate was able to survive exposure to 0.25% PI for 30 seconds (Table 1). | |
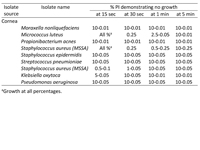
Table 1
Concentrations of povidone iodine (PI) resulting in no bacterial growth at various times, by isolate source
|
|
| Discussion | Prior in vitro research has shown dilute PI, approximately 0.25%, to produce the fastest killing time using nonocular bacterial isolates.(3) The largest series of clinical isolates from the eye, isolates from endophthalmitis cases, however, found greater bactericidal activity using higher concentrations of PI.(5) Our results are consistent with prior in vitro studies of PI, from nonocular sources, and suggest that PI has similar bactericidal action on pathologic bacteria from the ocular surface. No isolate was identified in which PI 10% had a faster killing time than PI 0.25%. Conversely, in 3 of 9 isolates the PI 0.25% solution had a faster killing time than both the 10% and 5% solutions.
In clinical use, the Shimada technique, which involves irrigating the ocular surface with dilute PI (0.25%) throughout cataract surgery has shown promise. A study of the anterior chamber contamination rate using this technique found a 0% contamination rate. This was significant compared with the 5% contamination rate found at the conclusion of surgery in the control group.(8) None of the pathologic clinical isolates in our study survived exposure to dilute PI solution (0.25%) for 30 seconds. The pharmacokinetics of PI on the ocular surface has not been studied, and a potential limitation of dilute PI solution is the decreased amount of total iodine.(9) While not bactericidal, the povidone-iodine complex does act as reservoir for free iodine, and in dilute solutions the supply of iodine may be exhausted prematurely. This could be important when frequent application is not practical.
Antiseptics generally, including PI, have applications beyond prophylaxis. They can be used as adjunct therapy to treat difficult to eradicate infections, to reduce the duration of antibiotic use, and as primary treatment of infection.(7,10,11) Additionally, compared with antibiotics, PI 1.25% use has been recently reported to be noninferior as a treatment for bacterial keratitis.(7) The low cost, wide availability, and simple preparation of PI may make it useful as a primary therapy in resource-limited settings. Expanded applications in settings where antibiotics are heavily used could focus on decreasing exposure to antibiotics and as adjunct therapy in multi- and pan-drug-resistant infections.
Resistance to PI has not been reported in conjunctival cultures, and repeated exposure to PI does not produce resistance or cross-resistance to antibiotics.(12,13) Future investigations of PI should include the study of low concentration frequently applied PI. Areas of further study include how PI solution can be used to decrease antibiotic exposure, the pharmacokinetics of PI on the ocular surface, and further study of pathologic eye isolates, particularly from endophthalmitis cases. Our study was an in vitro study and PI solutions could have different killing times on the ocular surface than in our experiment.
Literature Search
To identify the literature on the topics of PI, including basic science and clinical applications in ophthalmology, we performed a PubMed search, without date restriction, of English-language articles published prior to February 2018. The term povidone-iodine was searched in combination with the following terms: endophthalmitis, ophthalmology, prophylaxis, toxicity, pharmacokinetics, cataract surgery, resistance, and antisepsis protocol. The reference lists of relevant articles were reviewed to identify additional articles. | | | Acknowledgements | | The authors thank The Sight Society of Northeastern New York/Lions Eye Bank Albany for grant support to conduct this research | | | References | 1. Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg 1986;151:400-6.
2. Zamora JL, Price MF, Chuang P, Gentry LO. Inhibition of povidone-iodine’s bactericidal activity by common organic substances: an experimental study. Surgery 1985;98:25-9.
3. Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol 1982;15:635-9.
4. Shimada H, Nakashizuka H, Grzybowski A. Prevention and treatment of postoperative endophthalmitis using povidone-iodine. Curr Pharm Des 2017;23:574-85.
5. Hosseini H, Ashraf MJ, Saleh M, et al. Effect of povidone-iodine concentration and exposure time on bacteria isolated from endophthalmitis cases. J Cataract Refract Surg 2012;38:92-6.
6. Pelletier JS, Miller D, Liang B, Capriotti JA In vitro efficacy of a povidone-iodine 0.4% and dexamethasone 0.1% suspension against ocular pathogens. J Cataract Refract Surg, 2011. 37(4): p. 763-6.
7. Isenberg SJ, Apt L, Valenton M Prospective, randomized clinical trial of povidone-iodine 1.25% solution versus topical antibiotics for treatment of bacterial keratitis. Am J
Ophthalmol 2017;176:244-53.
8. Shimada H, Arai S, Nakashizuka H, Hattori T, Yuzawa M. Reduction of anterior chamber contamination rate after cataract surgery by intraoperative surface irrigation with 0.25% povidone-iodine. Am J Ophthalmol 2011;151:11-17.e1.
9. Koerner JC, George MJ, Meyer DR, Rosco MG, Habib MM. Povidone-iodine concentration and dosing in cataract surgery. Surv Ophthalmol 2018;63:862-8.
10. Riesgo AM, Park BK, Herrero CP, Yu S, Schwarzkopf R, Iorio R. Vancomycin povidone-iodine protocol improves survivorship of periprosthetic joint infection treated with irrigation and debridement. J Arthroplasty 2018;33:847-50.
11. Sharma D, Gathwala G, Shastri S. Chlorhexidine—a novel intervention to decrease the nursery stay and antibiotic exposure duration—randomized trial. J Matern Fetal
Neonatal Med, 2016;29:213-7.
12. Grzybowski A, Kanclerz P, Myers WG. The use of povidone-iodine in ophthalmology. Curr Opin Ophthalmol 2018 29:19-32.
13. Houang ET, Gilmore OJ, Reid C, et al. Absence of bacterial resistance to povidone iodine. J Clin Pathol. 1976;29:752-5. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in