|
|
 |
 |
 |
 |
|
|
The Enigma of Lenticular Oncology
Digital Journal of Ophthalmology 2001
Volume 7, Number 4
June 1, 2001
|
Printer Friendly
|



Gail M. Seigel, PhD | University at Buffalo, the State University of New York Ari Kummer | University of Rochester School of Medicine and Dentistry, Rochester, NY
|
|
|
| Abstract | Objective
It is a long-accepted dogma in Ophthalmology that the lens is a tumor-free tissue. Yet, in the lens, there is lifelong mitotic activity in the subcapsular epithelium. Therefore, there should be the potential for cellular transformation in these subcapsular epithelial cells. So, how can we EXPLAIN that, to our knowledge, no scientist has ever seen a naturally occurring primary tumor of the lens in vivo? In this review, we will discuss early work of Mann, von Hallermann, Courtois and others who addressed the issue of tumor resistance of the lens. | | | Introduction | "…any tissue or organ capable of cell division may be the site of a malignant neoplasm. Yet, the lens, as far as we know, in spite of the fact that mitoses occur in the subcapsular epithelium throughout life, never produces a cancer."-Ida Mann, 1948 [1].
In the 1940's, Ida Mann carried out studies to address the question of tumor-immunity in the lens. When rabbit lens in situ was micro-injected with highly carcinogenic 0.4% methylcholanthrene in liquid paraffin, the small puncture of the capsule sealed completely, and no change of the lens could be seen after ten months [1]. However, when mouse lenses were transferred to a vascularized site on the flank of inbred mice and treated with methylcholanthrene, a few lens-derived tumors were reported [1]. In 38 experiments, only three carcinomas of the subcapsular epithelium were mentioned, the remainder (26) were described as host-derived sarcomas or epitheliomas of the skin. In all cases of reported lens-derived tumor formation, the lens capsule was ruptured. Without modern antibody markers, we cannot exclude the possibility that the observed "carcinomas" and "sarcomas" may have represented epithelial hyperplasia, or a granular tissue response. In these early experiments, the putative lens-derived tumors did not occur in vivo, unless transplanted to a vascularized site, treated with a strong carcinogen, and the lens capsule ruptured [1]. The combination of these tumor-favorable conditions resulted in a small (8%) incidence of observed lens-derived tumor formation. Questions arise as to the explanation(s) for the low incidence of tumor formation. It is possible that lens tumors did not form because of low mitotic index of the cells being treated with methylcholanthrine. We also do not know the nature of the mutations induced by methyl cholanthrine in this model. Another possibility is that the lens or even the surrounding environment lacked the growth promoters necessary to induce transformed cells to divide.
Since the work of Ida Mann in 1948, the question of lens tumor resistance continues to be considered, taking INTO account the unique properties of the lens and lens capsule.
One unique property of the lens is the terminal differentiation of lens fiber cells accompanied by denucleation, a DNA fragmentation process akin to classical apoptosis [2, 3]. This process appears to involve a caspase 3-like cysteine protease [4], suggesting involvement of the apoptotic cycle in lens fiber differentation . The location and extent of apoptosis in the lens may contribute to the lack of tumor formation. One hypothesis is that this could occur through regulation of apoptotic pathway genes, such P53 and GADD45. Transgenic mouse models are being used to explore the role of apoptosis in lens cell cycle progression [5]. These kinds of studies may further address the potential role of apoptosis in the lifelong control of lens cell division. The apoptotic process associated with lens fiber cell denucleation still does not completely EXPLAIN the lack of naturally-occurring neoplastic transformation of the subcapsular epithelium. | | | Discussion | Lens subcapsular epithelial cells do not spontaneously transform in vivo
Spontaneous transformation of lens subcapsular epithelial cells in vivo has never been reported. Is this lack of neoplastic transformation a special property of the in vivo environment? In the late 1970's, Yves Courtois and colleagues studied the spontaneous transformation of bovine lens epithelial cells in vitro [6]. Under in vitro conditions, long-term lens epithelial cell cultures were shown to acquire unlimited growth characteristics. When transplanted INTO nude mice, these long-term cultured lens cells actively proliferated and formed tumors, which later regressed. When freshly dissociated lens epithelial cells were injected INTO nude mice at the same concentrations, no tumors were seen. In a later study by Messiaen and colleagues, cultures of mouse lens explants also acquired tumor-forming abilities after approximately 17 passages, but were not metastatic [7]. Therefore, these in vitro studies demonstrated the possibility that long-term culture conditions selected for the tumorigenic phenotype, altering growth characteristics through perturbation of the lens capsule and/or oncoviral infection. One question to consider is whether these lens cells were truly oncogenically transformed, or merely acquired a limited lifespan extension. Tumor regression could be explained by the fact that these cells grew to the end of their limited lifespan extension and then reached a point of senescence.
Other investigators have established lens epithelial cell lines, using SV40 virus in vitro [8]. Importantly, these results SHOW that lens epithelial cells, per se, are not resistant to oncogenic transformation under the appropriate circumstances. In addition transgenic mice were developed expressing MSV-SV 40 large T-construct [9] or murine alpha A-crystalline/SV 40 T antigen construct [10, 11]. In these transgenic mice, lens tumors of varying pathologies were observed. In the case of MSV-SV 40 large T-construct, one case of malignant transformation of the lens epithelium was found [9]. In the murine alpha A-crystalline/SV40 T antigen construct, the alpha T1 line developed fast growing, poorly differentiated lens tumors, whereas the alpha T2 line produced slow growing and well differentiated lens tumors [10]. However, these transgenic mice represent deviations FROM the natural lenticular environment. Perhaps there is something unique about the in vivo environment that provides protection against naturally-occurring neoplastic transformation of lens subcapsular epithelial cells.
Avascularity of the lens
The lens is an avascular tissue, acquiring its nutrients FROM aqueous and vitreous components (proteins, ions, growth factors) that pass through its semipermeable membrane, the lens capsule [12]. According to the work of Folkman [13] adequate vascular supply is essential for tumor progression.
As early as the 1970's J.Folkman [13] proposed that most solid tumors, regardless of origin, begin as a small population of cells dependent on nutrients that diffuse FROM surrounding tissues. Eventually small tumor colonies grow to a size where simple diffusion is insufficient to sustain growth. At this point, angiogenesis, fueled by tumor-derived factors, is necessary for further tumor growth. This would be the logical point where an avascular tissue, such as the lens, could confine potential tumors to a small size. However, even such preangiogenic colonies of neoplastic cells have not been described in the lens.
Therefore, it is possible that avascularity alone does not entirely EXPLAIN the lack of primary tumor formation in the lens. In another avascular ocular tissue, the cornea, Folkman and colleagues showed that inhibition of vascularization in an experimental tumor model could prevent tumor progression [14]. Yet, the cornea, despite its normally healthy state of avascularity, can still be invaded by advancing tumor tissue carrying its own angiogenic factors [14]. However, one important difference between the cornea and lens is that the cornea lacks a capsular barrier. Bowman's layer may serve as a type of corneal barrier. Primary tumors of the corneal stroma are virtually unknown, despite the fact that there is a high incidence of chromosomal abnormalities [15]. Therefore, it is likely that there is something about the ocular environment, and/or the presence of barriers that account for the lack of tumor formation in the lens.
The lens capsule as a chemo-mechanical barrier
The lens capsule is composed of collagen types I-IV [16, 17], laminin, entactin, heparan sulfate proteoglycan and fibronectin [18]. Interestingly, fragments of collagens (type XVIII and XV) make up endostatins [19], potent inhibitors of angiogenesis. One interesting speculation is that a collagen-derived endostatin-like molecule exists near and/or within the lens capsule as a protection mechanism against unwanted neovascularization. The presence of an endostatin-like molecule associated with the lens capsule would not only preserve the transparency of the lens but also inhibit the angiogenesis of tumors in close proximity. Another possibility is the presence of a lens capsule-associated cell growth inhibitor that prevents subcapsular epithelial cells FROM undergoing neoplastic transformation or uncontrolled growth in vivo. There is evidence in the literature that fragments of collagen type IV, the main component of the lens capsule, have the ability to suppress tumor cell growth [20], as well as inhibit activation of matrix metalloproteinases of tumor cells [21], believed to play a role in tumor invasiveness.
Some evidence in favor of the lens capsule as a chemo-mechanical barrier can be seen in Figure 1. Even highly invasive melanomas (Fig. 1 A, B) and retinoblastomas (Fig. 1C) that fill nearly the entire vitreous, demonstrate well-defined borders at the lens capsule interface. As generally seen in these tumors, there is no evidence of invasion, or direct contact with the lens capsule. Rather, the interface is filled with debris and fluid. It is tempting to speculate that the tumor is repelled by some type of chemo-mechanical property of the lens capsule. However, much more study is needed in this area.
In vitro experiments have demonstrated that tumor cells [22, 23] and leukocytes [24] do secrete proteolytic enzymes with the ability to digest lens capsule components. Yet, with the exception of one controversial paper by von Hallerman [25] in 1954, and another brief mention by Grossniklaus [26], invasion of the lens capsule by invading tumor cells appears to be an extremely rare event. Von Hallerman described lenticular invasion by melanosarcoma of the ciliary body and choroid [25]. A few presumptive tumor cells were described within a tear of the lens substance. These tumor cells showed no signs of mitotic activity. In another area, a few subcapsular tumor cells were shown with mitoses. Is this an unusual phenomenon, a fixation artifact, or an invasion of a non-intact lens capsule? The extreme rarity of lens tumor invasion leads to the possibility that the lens capsule itself or the surrounding ocular milieu inhibits digestion of the lens capsule by invasive tumor cells.
Based on our knowledge of the lens, there are several possible ways that tumor formation can be inhibited. Figure 2 schematically illustrates several hypotheses, including the presence of anti-angiogenic factors, growth inhibitors, as well as anti-lens capsule digestion factors. These factors may or may not be lens-derived, but rather in close proximity to the lens. One or more of these factors could play a role in the explanation of tumor-resistance of the lens.
Future experiments to test the capacity of the lens capsule as a chemo-mechanical barrier could be designed quite readily. The lens capsule could be placed in a chamber filled with vitreous like fluid. Tumor cells, such as retinoblastoma or melanoma cells, could be placed INTO the chamber on one side of the lens capsule. Then, the behavior of these tumor cells with respect to the lens capsule could be studied. Important questions could be addressed by adding anti-angiogenic factors, angiogenic factors, growth inhibitors, as well as anti-lens capsule digestion factors. The thickness of the lens capsule could also be tested as part of the barrier phenomenon. Another potential study, as suggested by an anonymous reviewer of this paper, would be an analysis of the effects of endostatins, angiogenesis factors, and pro/anti-apoptotic factors on the behavior of immortalized lens cells transplanted back INTO lens capsule ghosts, or alternatively, on the behavior of lentoid bodies derived FROM transdifferentiated RPE and retina in vitro (27).
Further, signaling processes between the lens capsule and the tumor cells could also be studied and might also reveal very interesting, new concepts as to why the lens seems to have such a strong barrier against tumor invasion.
The body of DATA FROM the past 50+ years shows that the enigma of lenticular oncology still exists and may have several possible explanations. For one, we now know that lens subcapsular epithelial cells themselves are not immune to neoplastic transformation in vitro, given the right circumstances. Therefore, it is the in vivo ocular environment and/or an intact lens capsule that seem to inhibit both the neoplastic transformation of lens subcapsular epithelial cells, as well as the breach of the lens capsule by invading tumor cells. Further characterization of the lens capsule and its ocular environment may shed more light on its unique role in anti-tumorigenesis. | |
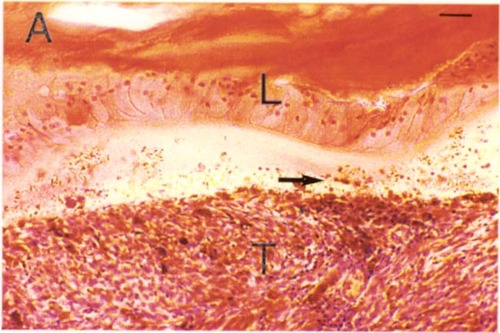
Figure 1a
Ocular melanoma (T) with numerous pigmented cell nests (arrowheads). Note the tumor-lens interface (arrow) and the intact lens capsule with subcapsular epithelial cells (L). H & E stain. Scale bar = 10 microns.
|
|
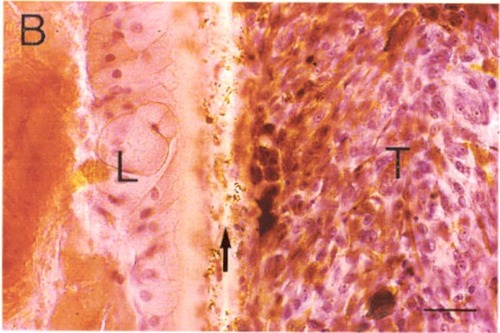
Panel 1b
Higher magnification of panel A. Note the debris and well defined tumor border (arrow). Scale bar = 10 microns.
|
|
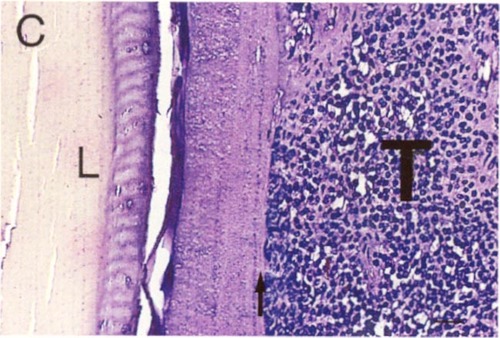
Figure 1c
Retinoblastoma (T) with well-defined border at the lens capsule interface (arrow). Note the fluid-filled space between the tumor and lens (L). H & E stain. Scale bar = 10 microns.
|
|
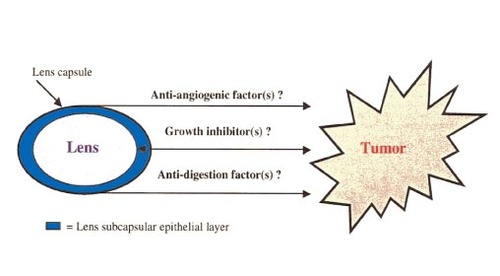
Figure 2
Schematic diagram of lens tumor resistance. Several hypotheses regarding the tumor-free state of the lens are shown. These hypotheses include the presence of anti-angiogenic factors, growth inhibitors, or anti-lens capsule digestion factors. These hypotheses are not mutually exclusive. In addition, note that the proposed growth inhibitors could affect tumor cell growth, as well as the growth/transformation of lens subcapsular epithelial cells.
|
|
| Acknowledgements | | The authors wish to thank Janet Wagner and Dr. S. Searl for assisting with histological specimens. We also thank the anonymous reviewer for helpful comments and suggestions. | | | References | 1. Mann I. Induction of an experimental tumor of the lens. Trans. Ophthalm. Soc. U. K. 67: 141-153 (1948).
2. Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation 61(2): 77-93 (1996).
3. Wride MA. Minireview: apoptosis as seen through a lens. Apoptosis 5(3): 203-9 (2000).
4. Ishizaki Y, Jacobson MD, Raff MC. A role for caspases in lens differentiation. J Cell Biol 140(1): 153-8 (1998).
5. Chen Q, Hung FC, Fromm L, Overbeek PA. Induction of Cell Cycle Entry and Cell Death in Postmitotic Lens Fiber Cells by Overexpression of E2F1 or E2F2. Invest Ophthalmol Vis Sci 41: 4223-4231 (2000).
6. Courtois Y, Simonneau L, Tassin J, Laurent MV, Malaise E. Spontaneous transformation of bovine lens epithelial cells. Differentiation 10: 23-30 (1978).
7. Messiaen L, Qian S, De Bruyne G, Boghaert E, Moens T, Rabaey M, Van Roy F, Mareel M. Spontaneous acquisition of tumorigenicity and invasiveness by mouse lens explant cells during culture in vitro. In Vitro Cell. Dev. Biol. 27A: 369-380 (1991).
8. Lenstra JA, Hukkelhoven MWCA, Groeneveld AA, Smits RAMM, Weterings PJJM, Bloemendal H. Gene expression of transformed lens cells. Exp Eye Res 35: 549-554 (1982).
9. Gotz W, Theuring F, Favor J, Herken R. Eye pathology in transgenic mice carrying a MSV-SV 40 large T-construct. Exp Eye Res 52(1): 41-9 (1991).
10. Nakamura T, Mahon KA, Miskin R, Dey A, Kuwubara T, Westphal H. Differentiation and oncogenesis: phenotypically DISTINCT lens tumors in transgenic mice. New Biol 1(2): 193-204 (1989).
11. Mahon KA, Chepelinsky AB, Khillan JS, Overbeek PA, Piatigorsky J, Westphal H. Science 235(4796): 1622-8 (1987).
12. Davson H. The lens. In: Physiology of the Eye, Fifth Edition, New York: Pergamon Press; p. 145-149 (1990).
13. Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. Exp Med. 133: 275-288 (1971).
14. Langer R, Brem H, Falterman K, Klein M, Folkman J. Isolation of a cartilage factor that inhibits tumor neovascularisation. Science 193: 70-72 (1976).
15. Pettenati MJ, Sweatt AJ, Lantz, Stanton CA, Reynolds J, Rao PN, Davis RM. The human cornea has a high incidence of acquired chromosome abnormalities. Hum Genet 101: 26-29 (1997).
16. Marshall GE, Konstas AG, Bechrakis NE, Lee WR. An immunoelectron microscopy study of the aged human lens capsule. Exp Eye Res 54(3): 393-401 (1992).
17. Schmut O. The organization of tissues of the eye by different collagen types. Albrecht Von Graefes Arch Klin Exp Ophthalmol 16; 207(3): 189-199 (1978).
18. Cammarata PR, Cantu-Crouch D, Oakford L, Morrill A. Macromolecular organization of bovine lens capsule. Tissue Cell 18(1): 83-97 (1986).
19. Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived FROM collagens XV and XVIII dffer in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol 301(5): 1179-90 (2000).
20. Kefalides NA, Monboisse JC, Bellon G, Ohno N, Ziaie Z, Shahan TA. Suppression of tumor cell growth by type IV collagen and a peptide FROM the NC1 domain of the alpha 3(IV) chain. Medicina (B Aires) 59(5 Pt 2): 553 (1999).
21. Pasco S, Han J, Gillery P, Bellon G, Maquart FX, Borel JP, Kefalides NA, Monboisse JC. A specific sequence of the noncollagenous domain of the alpha 3(IV) chain of type IV collagen inhibits expression and activation of matrix metalloproteinases by tumor cells. Cancer Res 60(2): 467-73 (2000).
22. Starkey JR, Hosick HL, Stanford DR, Liggitt HD. Interaction of metastatic tumor cells with bovine lens capsule basement membrane. Cancer Res 44(4): 1585-94 (1984).
23. Starkey JR, Stanford DR, Magnuson JA, Hammer S, Robertson NP, Gasic GJ. Comparison of basement membrane matrix degradation by purified proteases and by metastatic tumor cells. J Cell Biochem 35(1): 31-49 (1987).
24. Mainardi CL, Dixit SN, Kang AH. Degradation of type IV (basement membrane) collagen by a proteinase isolated FROM human polymorphonuclear leukocyte granules. J Biol Chem 255(11): 5435-41 (1980).
25. Von Hallermann W, Meisner G. Die umstrittene Tumorimmunitaet der Linse (translation: "The controversial tumor-immunity of the lens") Monatsblaetter fuer Augenheilkunde 124 Bd.: 159-164 (German) (1954).
26. Grossniklaus HE, MD, Zimmerman LE, MD, Kachmer ML, MD. Pleomorphic adenocarcinoma of the ciliary body. Ophthalmology 97: 763-768 (1990).
27. Pritchard DJ, Clayton RM, de Pomerai DI. 'Transdifferentiation' of chicken neural retina INTO lens and pigment epithelium in culture: controlling influences. J Embryol Exp Morphol 48:1-21 (1978). | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in