|
|
 |
 |
 |
 |
|
|
A retrospective study on the outcomes of Ahmed valve versus Ahmed valve combined with fluocinolone implant in uveitic glaucoma
Digital Journal of Ophthalmology 2017
Volume 23, Number 3
September 11, 2017
DOI: 10.5693/djo.01.2017.06.001
|
Printer Friendly
Download PDF |



Duriye D. Sevgi, MS | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts; Koc University School of Medicine, Istanbul, Turkey Samaneh Davoudi, MD | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts Katherine E. Talcott, MD | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts Heeyoon Cho, MD | Department of Ophthalmology, Hanyang University College of Medicine, Seoul, Republic of Korea Rong Guo, MS | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts Ann-Marie Lobo, MD | Department of Ophthalmology and Visual Science, University of Illinois, Chicago George N. Papaliodis, MD | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts Angela Turalba, MD | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts Lucia Sobrin, MD | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts Lucy Q. Shen, MD | Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts
|
|
|
| Abstract | Purpose
To compare the intraocular pressure (IOP) outcomes of Ahmed glaucoma valve (AGV) surgery alone versus AGV with fluocinolone implant in uveitic glaucoma patients.
Methods
We identified uveitic glaucoma patients with AGV surgery alone and AGV surgery combined with fluocinolone implant from the Massachusetts Eye and Ear Ocular Inflammation Database. Demographic information, visual acuity, and IOP were recorded at preoperative visits and 1, 6, and 12 months after surgery. Incidence of hypertensive phase, defined as an IOP of >21 mm Hg or use of additional treatment to lower IOP occurring any time between 7 days to 6 months postoperatively, was investigated. Multilevel mixed effects models were performed to compare the outcomes between groups.
Results
Eighteen eyes of 13 uveitic glaucoma patients with 1-year follow-up data were included. There were 11 eyes of 9 patients (mean age, 56.5 years; 63.6% male) in the AGV group and 7 eyes of 4 patients (mean age, 61.3 years; 71.4% male) in the AGV + fluocinolone group. There was no significant difference in visual acuity change at 1 year after surgery between groups (P = 0.25), although visual acuity improvement was significant in the AGV group (P = 0.01). The hypertensive phase occurred in 91% of AGV patients and 43% of AGV + fluocinolone patients (P = 0.30), with onset of 8-40 days (mean, 18 days) after surgery. IOP and number of glaucoma medications decreased at the 1-year postoperative visits in both the AGV group (P < 0.0001, P < 0.0001) and the AGV + fluocinolone group (P = 0.001, P < 0.0001). Compared to the AGV group, the AGV + fluocinolone group used fewer glaucoma medications (0.28 vs 1.30 [P = 0.01]) and had better inflammation control (P = 0.02). The surgical complication rates were similar between groups.
Conclusions
In uveitic glaucoma, AGV with fluocinolone achieves a similar, desired IOP control but with fewer glaucoma medications than AGV alone.
| | | Introduction | Elevated intraocular pressure (IOP), a significant risk factor for glaucoma, may be present in up to 46% of patients with uveitis, caused directly and indirectly by ocular inflammation.(1) Because IOP is uncontrolled on maximally tolerated medical therapy in 30% of uveitic glaucoma patients, surgical treatments are commonly employed, particularly trabeculectomy and placement of glaucoma drainage devices (GDDs).(2) The success rate of trabeculectomy ranges between 62% and 81% in this patient population. The Ahmed glaucoma valve (AGV; New World Medical, Rancho Cucamonga, CA), a commonly used GDD, has been shown to achieve adequate IOP control at 1 year in only 40.4% of uveitis patients.(3,4)
AGV surgery in nonuveitic glaucoma patients has been associated with hypotony in the first 10 or so postoperative days followed by a hypertensive phase (HP), which is characterized by a significant rise in IOP in the first 3 postoperative months.(5) It has been theorized that HP develops in the setting of postoperative inflammation, leading to congestion and fibrosis of the conjunctiva around the AGV plate, restricting aqueous flow.(6) It is particularly important to control IOP elevation in HP in patients with severe glaucomatous optic nerve damage.(5,6) Although no studies have specifically examined the prevalence of HP in uveitic glaucoma patients after AGV implantation, it has been observed frequently, presumably from persistent inflammation.
In nonuveitic patients, different approaches have been tried to minimize inflammation associated with AGV surgery to achieve better postoperative IOP control. Antimetabolites, such as 5-fluorouracil, mitomycin C, indomethacin, and colchicine have been associated with a lower incidence of HP.(7,8) Prolonged use of topical steroids, however, was associated with higher incidence of HP than use of topical NSAIDs.(9) Use of sub-Tenon’s triamcinolone acetonide injection during AGV surgery was shown to lower incidence of HP, but it did not improve long-term IOP control; additionally, it may be associated with more complications.(10)
The fluocinolone acetonide intravitreal implant (Retisert; Bausch & Lomb, Bridgewater, NJ) enables sustained corticosteroid release over approximately 3 years into the vitreous cavity.(11) It is approved for intraocular inflammation control in chronic, noninfectious intermediate, posterior, and panuveitis.(12,13) However, its use has been associated with corticosteroid-induced elevation of IOP in 75% of patients, with at least 37%-40% requiring surgery.(14-16)
The effect of a fluocinolone implant on IOP outcomes in uveitic patients undergoing glaucoma valve surgery has been examined in different studies. Three noncomparative case series, each no more than 15 patients, demonstrated the effectiveness of combined AGV and fluocinolone implant surgery for IOP and inflammation control.(17-19) One recent study compared combined AGV and fluocinolone implant surgery in uveitic glaucoma (22 eyes) to two other groups: AGV surgery alone in uveitic glaucoma (16 eyes) and AGV surgery alone in primary open-angle glaucoma (22 eyes).(20) The results were significant for longer successful IOP control in eyes that received the fluocinolone implant. Another recent study compared 17 uveitic glaucoma cases that received AGV + fluocinolone implant with 55 nonuveitic glaucoma controls that only received AGV.(21) The uveitic glaucoma group required fewer glaucoma medications and had lower IOPs compared to the nonuveitic glaucoma group.
None of these previous studies have examined the development of HP in detail. We hypothesize that the fluocinolone implant may be associated with lower incidence of HP in uveitic patients undergoing AGV surgery by minimizing intraocular inflammation in the immediate postoperative period after AGV surgery. The primary aim of the current study was to compare IOP outcomes of AGV alone and AGV combined with fluocinolone implant in uveitic glaucoma patients with a particular focus on the effect of the fluocinolone implant on the HP after AGV. We also aimed to examine secondary outcomes, including the use of glaucoma medications and inflammation control.
| | | Materials and Methods | This study was approved by the Massachusetts Eye and Ear Institutional Review Board, and all study procedures adhered to the Declaration of Helsinki. Medical records of patients with uveitis who underwent AGV surgery at the Massachusetts Eye and Ear between 2008 and 2013 were retrospectively reviewed. Inclusion criteria for the study were as follows: (1) diagnosis of uveitis by a fellowship-trained uveitis specialist; (2) diagnosis of glaucoma resulting directly or indirectly from uveitis by a fellowship-trained glaucoma specialist; (3) AGV surgery; and (4) postoperative follow-up of at least 1 year. All AGV surgeries were performed by glaucoma specialists using very similar techniques.(22) If both eyes met inclusion criteria, both were included.
Data Collection
From the preoperative visit for AGV surgery as well as post–AGV surgery visits at 1, 6, and 12 months, the following data were collected: demographic information, visual acuity, IOP, number of IOP-lowering medications, postoperative complications, etiology and duration of uveitis, medications used to control inflammation, and inflammation status. Snellen visual acuity measurements were converted to the logMAR scale. For visual acuity worse than 20/400, logMAR equivalencies were as follows: counting fingers, 1.98; hand motions, 2.28; light perception, 2.80.(23) All IOPs were measured by Goldmann applanation tonometry. Presence of inflammation was defined as anterior chamber cell of ?1+, vitreous haze of ?1, retinal vascular leakage on angiography, active chorioretinal lesions, or cystoid macular edema.(24)
Comparison Groups, Outcome Variables, and Covariates
Comparisons were made between eyes that had both AGV and fluocinolone implant placement (AGV + fluocinolone group) and those with AGV placement alone (AGV only). The primary outcome was the development of HP after AGV surgery. HP was defined as IOP of >21 mm Hg, use of additional glaucoma drops, or surgery to treat IOP between postoperative day 7 and the end of postoperative month 6. IOP was also examined as a continuous variable for maximal power. We also describe success of surgery as complete, qualified, and failure in each group. Surgery was considered a complete success when IOP of ?21 mm Hg was achieved without additional therapy. A qualified success was defined as IOP of ?21 mm Hg achieved with a single topical medication. Failure was defined as IOP of >21 mm Hg, IOP of ?21 mm Hg achieved with more than a single topical medication, and need for further surgery because of glaucoma. Other secondary outcomes included visual acuity, number of glaucoma medications, presence of inflammation, and complications at 1 year after AGV surgery. Covariate data, such as age, sex, number of antiglaucoma medications during the postoperative phase, and anti-inflammatory medications, were collected by uniform methods at each study visit. Continuous variables are reported as mean with standard deviation.
Statistical Analyses
We compared demographic and preoperative clinical variables between the AGV only and the AGV + fluocinolone groups. Categorical variables were compared using the mixed effect logistic regression, and continuous variables were compared with mixed effect linear regression. Mixed models were used for the analyses because we included both eyes for some patients and the outcomes at multiple time points were evaluated. These models are appropriate for research designs where data for participants are organized at more than one level. In this study, the units of analysis were the eyes (at a lower level), which are nested within patients, and time (at a higher level). Within each group, we also compared the different outcomes at 1 year postoperatively to preoperative values using mixed effect regression.
For the primary outcome of IOP after glaucoma surgery, we evaluated IOP change at 1 year and incidence of HP. Univariate and multivariate mixed effect linear regression were used to access percent change in IOP at 1 year, comparing the AGV only group and the AGV + flucinolone group. Multivariate regression model included age, sex, and number of antiglaucoma medications during the postoperative phase. A multivariate model examined the outcome of HP incidence with the same covariates.
To examine the secondary outcomes of visual acuity, number of glaucoma medications, and presence of inflammation, we also used mixed effect regression for both the univariate and multivariate analyses to compare the two groups. The multivariate regression models all included age and sex. The multivariate model for inflammation outcome also included the use of anti-inflammatory medications in the postoperative period.
For all analyses we used the subset of participants with complete information for the covariate of interest in that particular analysis to maximize the generalizability and power of the analysis. All analyses were performed using Stata/IC 12.1 (College Station, TX). | | | Results | Eighteen eyes of 13 patients met inclusion criteria: 11 eyes of 9 patients (mean age, 56.5 years; 63.6% male) in the AGV only group and 7 eyes of 4 patients (mean age, 61.3 years; 71.4% male) in the AGV + fluocinolone group. A total of 22.2% of eyes (40.0% in the Ahmed only group and 0.0% in the Ahmed + fluocinolone group [P = 0.07]) had prior glaucoma surgery; 72.2% of eyes (72.7% in the Ahmed only group and 71.4% in the Ahmed + fluocinolone group [P = 0.9]) were pseudophakic preoperatively. Seventeen AGV surgeries were performed using the FP-7 model and 1 AGV surgery using the S-2 (case 10, AGV + fluocinolone group). In the AGV + fluocinolone group, fluocinolone implant placement was concurrent with AGV surgery in 6 of 7 eyes. The remaining patient (case 10) in the group underwent cataract extraction with intraocular lens (IOL) implantation concurrently with fluocinolone implantation 4 months prior to AGV surgery. In 1 case (case 12) both eyes also had IOL implantation concurrently with fluocinolone implantation and AGV surgery (Table 1). All eyes (100%) that received fluocinolone implant were treated with systemic immunosuppressive therapy preoperatively compared to 4 of 11 eyes (36.4%) in the AGV only group (Table 1).
Table 2 shows the preoperative clinical characteristics of the two groups. Presence of inflammation at the preoperative visit was more common in the AGV + fluocinolone group (100%) compared to AGV only group (23% [P = 0.03]). Cup-to-disc ratio in the AGV only group was significantly larger than in the AGV + fluocinolone group (0.75 ± 0.19 vs 0.50 ± 0.23 [P = 0.03]). Other preoperative variables, including age, sex, visual acuity, IOP and number of antiglaucoma medications were not significantly different between the two groups.
Primary Outcome: IOP
Hypertensive phase at any time during the first 6 months occurred more frequently in the AGV only group (91%) compared to the AGV + fluocinolone group (43%), although this difference was not statistically significant in multilevel mixed analysis (P = 0.30). Mixed model regression compared IOP between groups at each time point after surgery and did not show a significant difference (Table 3). IOP was significantly decreased at the 1-year postoperative visits in both the AGV only and AGV + fluocinolone groups (P < 0.0001 and P = 0.001, resp.; Figure 1). There was no significant difference in the percent of IOP change between groups in the univariate and multivariate analyses (Table 4).
At 1 month after surgery, there was 54.5% failure, 27.3% qualified success, and 18.2% complete success in the AGV only group compared to 14.3% failure, 14.3% qualified success, and 71.4% complete successes in the AGV+ fluocinolone group (P = 0.11). At 6 months’ follow-up, the AVG only group had 54.5% failure, 27.3% qualified success, and 18.2% complete success compared to 0% failure, 28.6% qualified success, and 71.4% complete success in the AGV+ fluocinolone group (P = 0.91). At 1 year, the failure rate was 63.6%, the qualified success rate was 27.3%, and the complete success rate was 9.1% in the AVG only group compared to 0% failure, 28.6% qualified success, and 71.4% complete success in AGV+ fluocinolone group (P = 0.94).
Secondary Outcomes: Visual Acuity, Number of Glaucoma Medications, and Inflammation
Visual acuity had improved in both groups at the 1-year postoperative visit, but the improvement was only significant in the AGV group (P = 0.01). Comparing the groups to each other, there was no significant difference in the visual acuity change at 1 year after surgery (P = 0.25, Table 4).
The number of glaucoma medications was significantly decreased at the 1-year postoperative visit in both groups (P < 0.001 for both). The AGV + fluocinolone group used fewer antiglaucoma medications at the 1-year postoperative visit than the AGV only group (mean, 0.28 ± 0.48 vs 1.30 ± 1.01 [P = 0.01]; Table 3).
Compared to preoperative status, the presence of inflammation was significantly less at one year in the AGV + fluocinolone implant group (P = 0.006), but did not change significantly in the AGV only group (P = 0.20). The presence of inflammation at 1 year was more common in the AGV only group (27.3%) compared to the AGV + fluocinolone implant group (9.1%). The odds of having inflammation during the first postoperative year was 0.49 (P = 0.02) in the AGV + fluocinolone group compared to AGV only group in the multivariate model, after controlling for age, sex, and use of anti-inflammatory medications (Table 4, Figure 2).
Complications
Complications requiring additional surgery occurred in 3 eyes (1 in the AGV only group [9.1%] and 2 in the AGV + fluocinolone group [28.5%], P = 0.57). In the AGV only group, 1 eye underwent a second AGV placement approximately 1 year after the initial surgery for persistently high IOP (28 mm Hg) despite maximal medical treatment (case 6). In the AGV + fluocinolone group, 2 eyes of the same patient developed tube erosions without infection or hypotony at 4 and 7 months postoperatively (case 12). | |
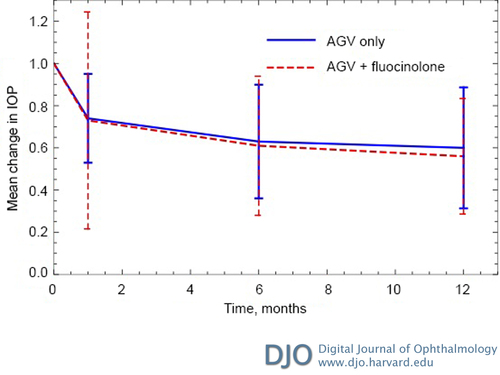
Figure 1
Change in intraocular pressure (IOP) postoperatively. Mean change in IOP was determined from the IOP at each postoperative time point divided by the preoperative IOP. The final change in IOP at postoperative month 12 were 40% for the Ahmed glaucoma valve (AGV) group and 52% for the AVG + fluocinolone group (P = 0.15). Error bars represent standard errors at each point time.
|
|

Figure 2
Percent of eyes with inflammation during 1-year follow-up. The percent of eyes with inflammation was unchanged in the AGV group (27% preoperatively, 9.1% at 12 months postoperatively 12 [P = 0.20]) and significantly improved in the AGV + fluocinolone group (100% preoperatively, 0% at 12 postoperatively 12 [P = 0.006]).
|
|
| Discussion | Glaucoma is a known complication of uveitis, affecting up to 46% of patients.(1) AGV surgery is commonly performed when glaucoma cannot be controlled with medical management. Presence of inflammation and steroid use in uveitic glaucoma patients may contribute to poor outcomes following AGV surgery, such as HP, tube failure, and tube erosion. This study compared uveitis patients who underwent AGV surgery alone to those who had both AGV and fluocinolone implant surgery. We found that both the AGV only and the AGV + fluocinolone groups had significant IOP reduction at 1 year after AGV surgery. The presence of a fluocinolone implant did not lead to further decrease in the IOP at 1 year; nor did it diminish the risk of a hypertensive phase in a multivariate model. However, the AGV + fluocinolone group used fewer glaucoma medications and had less intraocular inflammation at 1 year. The complete success was higher in the AGV + fluocinolone group compared to the AGV only group at any of the postoperative time points, although this difference did not reach statistically significance.
Two studies have compared outcomes of AGV surgery combined with fluocinolone implant and AGV only.(21,20) As in the present study, Moore et al found no significant difference between the average IOP and IOP changes between groups.(20) Contrary to our study, however, they did not find a difference in the number of glaucoma medications between groups at 12 months.(20) Another study compared the outcomes of AGV + fluocinolone implant surgery in eyes with uveitic glaucoma to AGV alone in eyes without uveitis and found lower mean IOP and less glaucoma medication at 1 year in the AGV + fluocinolone group.(21) Our study is the first to show that AGV + fluocinolone implant significantly reduced the number of glaucoma medications used at 1 year compared to AGV alone in patients with uveitic glaucoma.
Furthermore, this is the first study to examine the effects of fluocinolone implantation on the rate of HP after AGV in uveitis patients. The definition of HP in this study was not based on IOP parameter alone, because glaucoma medications were started at lower IOP in an effort to prevent significant IOP spikes.(25) This is particularly true for patients who developed HP in the contralateral eye (cases 1, 5, 11; Table 1). Although we did not detect a significant difference in HP between groups, the AGV + fluocinolone group had a lower percentage of HP and lower mean IOP at final follow-up than the AGV only group. A limitation of this study, however, is the small sample size, which may not be adequately powered to detect this difference between the two groups. The estimated sample size was 20 subjects in each group to detect the difference by considering that the type I error rate = 0.05, the mean and standard deviation (SD) of IOP in each group after 12 months (mean, ± 6.6 in AGV only group; mean, 11.3 ± 4.7 in the AGV+ fluocinolone group) and a desired power of 80%. Furthermore, all 7 eyes in the AGV + fluocinolone group, despite being on systemic immunosuppression within 6 months preoperatively, had inflammation at baseline compared to 3 of 11 eyes in AGV only group (P = 0.03). This baseline difference is a poor prognostic factor for AGV + fluocinolone, because inflammation is associated with worse IOP outcome and higher failure rates after glaucoma drainage device surgeries.(26,27) Hence, the lack of difference in HP and IOP control postoperatively between groups is suggestive of a beneficial effect of AVG + fluocinolone.
We did observe that the AGV + fluocinolone group used significantly fewer glaucoma medications 1 year after surgery than the AGV only group. Furthermore, none of the eyes in the AGV + fluocinolone group required additional glaucoma surgery for IOP control, compared to 1 eye in the AGV group (9.1%). These findings suggest that sustained inflammation control achieved with fluocinolone implant improved the surgical outcome of AGV surgery in patients with uveitic glaucoma.
At 1 year postoperatively, there was no significant difference in visual acuity between groups. The improvement in visual acuity in the AGV only group may have been influenced by other surgeries during the year, including penetrating keratoplasty (case 4) and IOL exchange (case 10, case 12 both eyes). The postoperative visual acuity was not significantly different from preoperative visual acuity in the AGV + fluocinolone group. In this respect, our results differed from those of Moore et al, which showed a significant improvement in this group; however, this improvement in visual acuity may be explained by the higher percent (59%) of simultaneous cataract extraction.(20) In the present study only 2 eyes (28%) had concurrent cataract extraction and IOL implantation in the AGV + fluocinolone group (case 12).
It is important to note that 1 patient (case 12) in the AGV + fluocinolone group developed tube erosion in both eyes (28.5%). The surgeries were performed in a similar manner to other eyes in the group, and the tube was initially covered with Tutoplast pericardium (Katena, Denville, NJ). It is possible, nevertheless, that the fluocinolone implant caused impaired wound healing leading to gradual conjunctiva breakdown. The rate of tube erosion in our study is higher than has been previously reported (1.7%-13.3%).(28,29) On the other hand, given that tube erosions were only observed in the same and only patient, it is also possible that the patient’s underlying systemic disease (sarcoidosis with ocular involvement) and/or dry eyes played a role in the erosions.
This study has several limitations. It is retrospective, nonrandomized, and has a relatively small sample size, which may have resulted in large confidence intervals in the multivariate analysis. The follow-up time was only 1 year. Because fluocinolone implant releases steroid for approximately 30 months, longer follow-up times are needed for a more comprehensive assessment. The decision to perform fluocinolone implantation surgery as well as the addition of glaucoma medications were at the discretion of the providers, which could be associated with selection bias. In addition, the two groups were not matched in preoperative inflammation status or glaucoma status.
In conclusion, this study found that IOP control was achieved with AGV and fluocinolone implants in uveitic glaucoma. Although the incidence of hypertensive phase was not significantly lowered, this surgery is associated with fewer glaucoma medications postoperatively and better inflammation control compared to AGV alone. Further investigations are necessary to better assess the effects of sustained inflammation control on the surgical treatment of uveitic glaucoma. | | | References | 1. Herbert HM, Viswanathan A, Jackson H, Lightman SL. Risk factors for elevated intraocular pressure in uveitis. J Glaucoma 2004;13:96-9.
2. Kalin-Hajdu E, Hammamji K, Gagne S, Harasymowycz P. Outcome of viscodilation and tensioning of Schlemm’s canal for uveitic glaucoma. Can J Ophthalmol 2014;49:414-9.
3. Yakin M, Eksioglu U, Sungur G, Satana B, Demirok G, Ornek F. Short-term to long-term results of Ahmed Glaucoma Valve implantation for uveitic glaucoma secondary to Behçet disease. J Glaucoma 2016;6:20-6.
4. Gregory AC 2nd, Zhang MM, Rapoport Y, Ling JD, Kuchtey RW. Racial influences of uveitic glaucoma: consolidation of current knowledge of diagnosis and treatment. Semin Ophthalmol 2016;31:400-4.
5. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology 1998;105:1968-76.
6. Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol 2003;136:1001-8.
7. Amoozgar B, Lin SC, Han Y, Kuo J. A role for antimetabolites in glaucoma tube surgery: current evidence and future directions. Curr Opin Ophthalmol 2016;27:164-9.
8. Yu YS, Youn DH. The effect of colchicine on fibroblast proliferation after glaucoma filtering surgery. Korean J Ophthalmol 1987;1(2):59-71.
9. Yuen D, Buys Y, Jin YP, Alasbali T, Smith M, Trope GE. Corticosteroids versus NSAIDs on intraocular pressure and the hypertensive phase after Ahmed glaucoma valve surgery. J Glaucoma 2011;20:439-44.
10. Teixeira SH, Doi LM, Freitas Silva AL, et al. Silicone Ahmed glaucoma valve with and without intravitreal triamcinolone acetonide for neovascular glaucoma: randomized clinical trial. J Glaucoma 2012;21:342-8.
11. Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. Ocular pharmacokinetics of fluocinolone acetonide after Retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther 2004;20:269-75.
12. Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW; Fluocinolone Acetonide Study Group. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology 2010;117:567-75,575.e1.
13. Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA; Multicenter Uveitis Steroid Treatment Trial Research Group. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol 2010;149:550-61.e10.
14. Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol 2008;126:1191-1201.
15. Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol 2006;17:163-7.
16. Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol 2007;125:1478-85.
17. Ahmad ZM, Hughes BA, Abrams GW, Mahmoud TH. Combined posterior chamber intraocular lens, vitrectomy, Retisert, and pars plana tube in noninfectious uveitis. Arch Ophthalmol 2012;130:908-13.
18. Chang IT, Gupta D, Slabaugh MA, Vemulakonda GA, Chen PP. Combined Ahmed Glaucoma Valve placement, intravitreal fluocinolone acetonide implantation and cataract extraction for chronic uveitis. J Glaucoma 2016;25:842-6.
19. Malone PE, Herndon LW, Muir KW, Jaffe GJ. Combined fluocinolone acetonide intravitreal insertion and glaucoma drainage device placement for chronic uveitis and glaucoma. Am J Ophthalmol 2010; 149:800-806.e1.
20. Moore DB, Stinnett S, Jaffe GJ, Asrani S. Improved surgical success of combined glaucoma tube shunt and Retisert((R)) implantation in uveitic eyes: a retrospective study. Ophthalmol Ther 2015;4:103-13.
21. Hennein L, Hou J, Stewart JM, et al. Comparison of surgical outcome after Ahmed valve implantation for patients with and without fluocinolone intravitreal implant (Retisert). J Glaucoma 2016;25:e772-6.
22. Barton K, Gedde SJ, Budenz DL, et al. The Ahmed Baerveldt Comparison Study methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology 2011;118:435-42.
23. Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol 2009;247:137-42.
24. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature Working Group. Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol 2005;140:509-16.
25. Law SK, Kornmann HL, Giaconi JA, Kwong A, Tran E, Caprioli J. Early aqueous suppressant therapy on hypertensive phase following glaucoma drainage device procedure: a randomized prospective trial. J Glaucoma 2016;25:248-57.
26. Ozdal PC, Vianna RN, Deschenes J. Ahmed valve implantation in glaucoma secondary to chronic uveitis. Eye (Lond) 2006;20:178-83.
27. Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol 2005;50:48-60.
28. Rachmiel R, Trope GE, Buys YM, Flanagan JG, Chipman ML. Ahmed glaucoma valve implantation in uveitic glaucoma versus open-angle glaucoma patients. Can J Ophthalmol 2008;43:462-7.
29. Papadaki TG, Zacharopoulos IP, Pasquale LR, Christen WB, Netland PA, Foster CS. Long-term results of Ahmed glaucoma valve implantation for uveitic glaucoma. Am J Ophthalmol 2007;144:62-9. | |

Table 1
Clinical description of patients in the Ahmed and Ahmed + fluocinolone groups
|
|

Table 2
Preoperative clinical characteristics
|
|
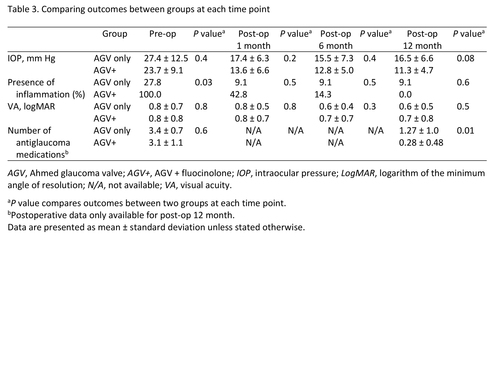
Table 3
Comparing outcomes between groups at each time point
|
|
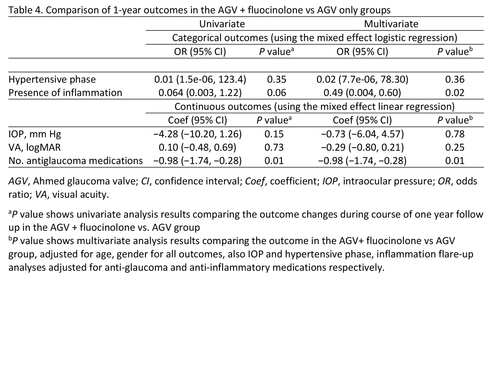
Table 4
Comparison of 1-year outcomes in the AGV + fluocinolone vs AGV only groups
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in