|
|
 |
 |
 |
 |
|
|
Retinal detachments after Boston Keratoprosthesis: incidence, predisposing factors, and visual outcomes
Digital Journal of Ophthalmology 2015
Volume 21, Number 4
December 21, 2015
DOI: 10.5693/djo.01.2015.10.001
|
Printer Friendly
Download PDF |


 Maria Stephanie R. Jardeleza, MD
Maria Stephanie R. Jardeleza, MD | Department of Ophthalmology, University of Texas Health Science Center in San Antonio–Texas Diabetes Institute, San Antonio, Texas Marc-Andre Rheaume, MD | Retina Service, Department of Ophthalmology, University of Montreal, Quebec, Canada James Chodosh, MD, MPH | Cornea Service, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts Anne Marie Lane, MPH | Retina Service, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts Claes H. Dohlman, MD, PhD | Cornea Service, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts
|
|
|
| Abstract | Purpose
To determine the rates, predisposing factors, and visual outcomes of retinal detachment (RD) after Boston Keratoprosthesis (KPro) implantation.
Methods
In this noncomparative, interventional case series, the medical records of 170 patients (205 eyes) who underwent Boston type 1 and type 2 KPro implantation at the Massachusetts Eye and Ear Infirmary between April 1993 and June 2009 were retrospectively reviewed. Incidence and annual rates of RD were calculated, and the roles of possible predictive factors for RD after KPro were investigated. Main outcome measures were rates of and risk factors for RD, visual acuity after RD, and surgical outcomes after repair.
Results
Sterile vitritis and autoimmune systemic disease significantly predisposed patients to RD after KPro placement. Of patients who developed RD after implantation, 50% progressed to visual acuity of no light perception despite surgical repair.
Conclusions
Inflammation plays a major role in RD development after KPro implantation. Patients with predisposing factors should be advised of the high rates of RD and comanaged with a vitreoretinal specialist. | | | Introduction | The Boston Keratoprosthesis (KPro) is a viable option after multiple failed corneal grafts or in patients who are poor prognostic candidates for primary penetrating keratoplasty.(1,2) The number of keratoprosthetic procedures performed in the United States has increased greatly over the past several years.(3) Keratoprosthesis implantation offers the chance of functional visual acuity in patients with otherwise “end-stage” eyes.(2-4) Recent studies report postoperative visual acuity of better than 20/40 in up to 25% of patients undergoing the procedure.(1,5) However, secondary comorbidities, such as advanced glaucoma and vitreoretinal pathology, often lead to severe visual loss and failure of visual acuity to improve after keratoprosthesis implantation.(1) Retinal detachments, along with cystoid macular edema, macular epiretinal membranes, and retroprosthetic vitreous membranes, are vitreoretinal complications that can impair visual acuity after implantation.(6,7)
Retinal detachment (RD) after KPro implantation can be devastating for the patient and remains very challenging to manage. Knowledge of predisposing factors for RD in certain patient populations can guide surgical planning and patient follow-up. Awareness of surgical techniques or outpatient procedures that either prevent or predispose to RDs can improve long-term visual outcomes in patients undergoing keratoprosthesis placements. | | | Materials and Methods | This study was approved by the Institutional Review Board at the Massachusetts Eye and Ear Infirmary. The medical records of 170 patients (205 eyes) who had undergone Boston type 1 and type 2 keratoprosthesis implantation by the same surgeon (CHD) at the Massachusetts Eye and Ear Infirmary between April 1993 and June 2009 were reviewed.
The incidence and annual rates of RD were calculated. Rates of RD between patients who underwent KPro implantation for systemic versus local factors were compared. The role of the following possible predictive factors for RD were investigated: indication for KPro implantation (local vs systemic disease), presence of retroprosthetic membrane (RPM), lens status (aphakia vs pseudophakia), history of Nd:YAG laser, sterile vitritis, endophthalmitis, glaucoma tube shunt implantation, history of anterior vitrectomy at the time of implantation, and development of a leak after KPro. Wilcoxon rank sum test and the Fisher exact test were used to determine significant differences between patients who developed RD and the rest of the study population. Correlation between significant risk factors for retinal detachment was determined using the Fisher exact test.
Cox regression analysis was completed in a stepwise fashion. All factors found to be significantly associated with RD in univariate analysis were entered into a single (full/multivariate) model. Factors that were not significantly associated with RD were dropped from the model.
Available operative reports on patients who developed RDs were reviewed. RD repair was performed by vitreoretinal surgeons of the Mass Eye and Ear. Anatomical and visual outcomes of RDs, those that were repaired and otherwise, were analyzed. Details of RD timing after KPro implantation were noted. | | | Results | The mean age at first KPro implantation was 59.6 years (range, 17-94 years), with each eye being followed for an average period of 3.9 years (range, 1 month to 16.6 years). The mean number of KPro implanted per eye was 1.3 (range, 1-4). A type 1 KPro was placed in 177 eyes (86.3%); a type 2 KPro, in 28 eyes (13.7%). Indications for surgery in eyes that developed RD were as follows: local pathology, 60%; autoimmune/systemic disease, 31%; chemical burn, 9%.
Forty-four eyes of 38 patients (21.5%) developed RD after KPro implantation, with a mean follow-up of 5.4 years. The mean time to RD after implantation was 30.5 months (range, 0.7-113.5 months). There was no difference between the mean age of patients that developed RD and those that did not (57.2 vs 60.6 years; P = 0.35). Annual rates and cumulative rates of RD are presented in Table 1.
The majority of RDs occurred in patients with underlying autoimmune systemic disease (Table 2). Due to the small number of patients undergoing KPro implantation for chemical burns, this category was combined with those patients that underwent KPro placement for non-autoimmune etiology and grouped under “local factors.”
A significantly higher incidence of RD occurred in patients with RPM, sterile vitritis, leakage after implantation and underlying autoimmune systemic disease (Figure 1). The number of KPro implantations per eye, development of endophthalmitis after KPro, lens status, history of Nd:YAG laser, and tube shunt placement were also predictive of RD (Figure 2). Vitrectomy during KPro implantation, however, did not appear to be a significant risk factor (P = 0.9).
Cox univariate regression analysis, however, demonstrated that only the presence of RPM (RR = 3.3; P = 0.007), sterile vitritis (RR = 2.9; P = 0.001), endophthalmitis (RR = 2.4; P = 0.05), development of a leak after KPro implantation (RR = 2.5; P = 0.004), and underlying autoimmune systemic disease (RR = 3.5; P < 0.001) were significantly associated with an increased risk of developing an RD after KPro. Presence of an RPM, sterile vitritis, and underlying autoimmune disease were all strongly correlated. Adjusting for the correlation among these 3 factors using multivariate analysis, sterile vitritis and underlying autoimmune systemic disease remained strongly associated with RD occurrence after placement (Table 3).
Surgical reports were available for 37 of the 44 eyes that developed an RD after KPro. At the time of diagnosis, 27 of 37 eyes (73%) were considered irreparable. Visual outcomes of RD after implantation were as follows: 50% of patients deteriorated to no light perception, and only 11% of patients retained visual acuity of at least 20/400. | |
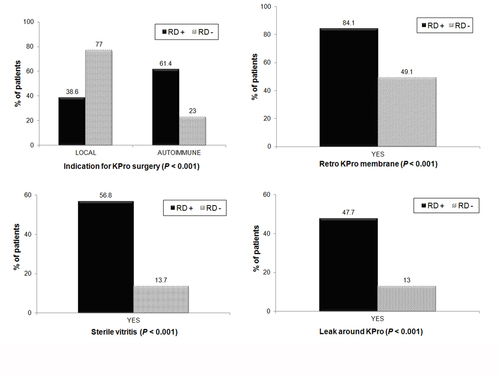
Figure 1
Factors strongly predictive of a retinal detachment after KPro. The black bars in the figures indicate the percentage of retinal detachment patients with the indicated risk factors.
|
|
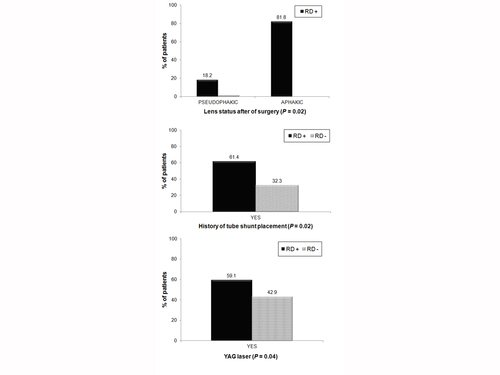
Figure 2
Factors moderately predictive of a retinal detachment after KPro. The black bars in the figures indicate the percentage of retinal detachment (RD) patients with the indicated risk factors.
|
|
| Discussion | Significant advances have been made in improving visual outcomes and long-term stability of the Boston Keratoprosthesis.(1,3) Retinal detachments following keratoprosthesis placement, however, remain a major cause of ocular morbidity and suboptimal vision following implantation. Attempts at repair pose surgical challenges unique to eyes containing a keratoprosthesis.(6)
We present the largest series on RD after Boston type 1 and type 2 KPro placement. Our series is also the largest study investigating peri- and postoperative factors predisposing to RD after implantation. Compared with other series,(1,5,7,19,20) ours shows a much higher incidence of RD, most likely because of the relatively large number of patients with longer follow-up and the higher incidence of autoimmune cases.
Lens Status
The majority of patients in our study who developed RD had their posterior capsule removed during implantation. Although aphakia has been reported to predispose to a higher incidence of retinal detachments,(8) it was not shown after additional regression analysis to be a significant contributory factor to RD development after Boston KPro implantation. Violation of the posterior capsule and vitreous loss are predisposing factors to RD.(9) In contrast, Bradley et al reported no RDs in a series of 30 eyes, of which 28 were pseudophakic at the end of implantation.(5)
Retroprosthetic Membrane and/or Nd:YAG Capsulotomy
Formation of a retroprosthetic membrane is one of the most common visually limiting posterior segment complications of KPro placement.(3,5,7,10) In our series, 84% of patients that developed an RD after implantation had RPMs, which are managed either by Nd:YAG capsulotomy or, in cases of recalcitrant membranes, by removal via pars plana vitrectomy.(3,5,6,20) Nd:YAG capsulotomy has been linked with a higher incidence of RD in pseudophakic eyes.(11,12) Thus, 58% of RD patients had a history of Nd:YAG laser capsulotomy prior to developing an RD. Both RPM formation and Nd:YAG capsulotomy were significantly associated with RD development in our series. Goldman et al reported a significantly greater percentage of RDs in eyes undergoing Nd:YAG capsulotomy for RPM in their series.(19) This is in contradiction to a series by Chak and Aquavella,(13) which did not report any RD occurring in association with RPM formation or after Nd:YAG capsulotomy. We recommend that KPro patients with RPM formation and those that undergo Nd:YAG capsulotomies be followed closely in the immediate postoperative period for RD. It is unclear in our series whether pars plana removal of the membrane decreases the likelihood of RD in comparison with Nd:YAG capsulotomy.
The Role of Inflammation
Patients were divided into three groups for analysis—the autoimmune group (Stevens-Johnson syndrome, ocular cicatricial pemphigoid, etc), chemical burns group, and non-autoimmune group (herpetic keratitis, pseudophakia/aphakic bullous keratopathy, etc); however, the number of chemical burn patients was small, and these patients were grouped with the non-autoimmune patients. The non-autoimmune group constituted the largest number of patients. The most striking result of the present study is that RD following KPro implantation is more than 4 times more frequent in patients with autoimmune disease (where KPro is generally not recommended) than in non-autoimmune cases. Compared to other series,(3,5,7,10) the present series had a much higher number of patients undergoing KPro implantation for ocular surface disease associated with autoimmune conditions.
This highlights the role that inflammation plays in the development of RD after KPro. Proliferative vitreoretinopathy and vitreoretinal adhesions are sequelae of chronic inflammation and may play a role in causing RD.(6) Our study demonstrates that sterile vitritis after implantation significantly predisposed our patients to RD. The incidence of sterile vitritis in other series was low,(3,5,7) and no cases were reported to be associated with RD. RPM formation, another predisposing factor to RD in our series, has also been attributed to inflammation(14) and associated with autoimmune disease.(15,16) We have demonstrated a strong correlation among the 3 factors most strongly associated with RD after KPro: sterile vitritis, RPM formation, and history of autoimmune disease. Accounting for these correlations, sterile vitritis and indication for surgery (autoimmune vs local disease) appear to be the only factors strongly predictive for development of an RD. Based on our records, administration of intracameral steroids during KPro implantation or periocular steroids in the acute postoperative period in an attempt to control inflammation does not appear to be protective against detachments.
Patients with underlying autoimmune systemic disorders and possibly those that develop sterile vitritis after implantation should be followed closely for RD after keratoprosthetic surgery and warrant close comanagement with a vitreoretinal specialist.
Concomitant Procedures
Glaucoma tube shunt placement is a commonly performed surgery in KPro implantation patients.(3,7,16,17) This can be performed before, during, or after implantation. Our study indicates that this can predispose to RD (Figure 2); nevertheless, there is usually no choice but to proceed with the shunt placement in patients with recalcitrant glaucoma after KPro.
Vitrectomy
Anterior vitrectomy is often performed in conjunction with KPro implantation, especially if the patient is to be left aphakic after the surgery.(3,7) We investigated whether a concomitant anterior vitrectomy could be protective against RD. The premise was that removal of the vitreous might decrease the likelihood that vitreous traction contributed to RD. However, no relationship between concomitant vitrectomy and RD was seen in our series.
Surgical and Visual Outcomes of Retinal Detachment Repair
The majority of KPro patients diagnosed with RD in this series were considered “irreparable” at the time of diagnosis—usually because the retina was stiff and fibrotic, with severe proliferative vitreoretinopathy at the time of examination. Regardless of repair, visual outcome was dismal in patients that developed RD. This markedly contrasted with overall good visual outcomes reported in several series after KPro implantation.(1,3,5,7,10) Recent advances in smaller gauge vitreoretinal surgery and more modern posterior segment techniques have contributed to better surgical outcomes of pars plana vitrectomy in patients with KPro implantation.(20)
Other Factors Associated with RD
We have also demonstrated that development of infectious endophthalmitis and/or a leak after KPro implantation predisposes to RD formation. Both are now relatively rare complications,(5,7,10) the former due to the advent of prophylactic antibiotics used indefinitely after implantation.(18)
This study has several limitations. No attempts were made by the authors to correlate the adequacy of systemic immunosuppressant therapy with the incidence of RD outcomes in patients with systemic autoimmune disease. Surgical reports were also not available for 9 patients who developed RD after KPro implantation. This could account for the surgical discrepancy in the number of RD repairs compared to an earlier series from our institution by Ray et al.(6)
The present study represents by far the largest available cohort of RD after KPro. It includes a number of patients from the pioneering days of KPro in the 1990s, when management was still quite uncertain. In addition, a large percentage of patients had autoimmune diseases (>30%), and the cohort of chemical burns was very severe. These factors probably account for our higher RD rate compared with other published series.(5,7,19,21) However, the size of the patient group has allowed us to analyze the risk factors in some depth. Retroprosthetic membrane, leak, autoimmune status, and sterile vitritis were related to increased RD risk. Degree of inflammation was a strong common denominator. Surprisingly, vitrectomy at the time of KPro surgery did not seem to influence the incidence of RD. Our data showed that RD developed in 42% of eyes from patients with autoimmune disease and only 12% of eyes with local conditions during a mean follow-up of 5.4 years. By contrast, other studies have shown much lower RD complication rate.(13,21) Our more recent experience is similar.
This review has taught us the long-term risks of KPro implantation in autoimmune patients and those with severely inflamed eyes. It also illustrates the importance of ongoing collaboration with retina specialists as well as the necessity of continued research on suppression of inflammation. For patient safety it seems reasonable for the moment to advise against routine KPro surgery in patients with autoimmune diseases and uveitis, outside rigorous studies in academic settings.
Literature Search
PubMed was search on December 4, 2015, without language restriction, using the following terms in combination: Boston Keratoprosthesis, retinal detachment, complications, vitreoretinal, and posterior segment. | | | Acknowledgements | Presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL, April 27-May 1, 2008; and at the Annual Meeting of the Association for Research in Vision and Ophthalmology Meeting, Fort Lauderdale, Florida, May 3-7, 2009.
All authors are, or were, full-time employees of Massachusetts Eye and Ear Infirmary (a not-for profit organization), which is the manufacturer of the Boston Keratoprosthesis.
The authors thank Lucy Young, MD, PhD, for her assistance and input in the preparation of this manuscript. | | | References | 1. Zerbe BL, Belin MW, Ciolino JB. Results from the Multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology 2006;113:1779-84.
2. Sayegh RR, Ang LPK, Foster S, Dohlman CH. The Boston Keratoprosthesis in Stevens Johnson syndrome. Am J Ophthalmol 2008;145:438-44.
3. Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type 1 keratoprosthesis. Improving outcomes and expanding indications. Ophthalmology 2009;116:640-51.
4. Harissi-Dagher M and Dohlman CH. The Boston Keratoprosthesis in severe ocular trauma. Can J Ophthalmol 2008;43:165-9.
5. Bradley JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type 1 keratoprosthesis: the University of California Davis experience. Cornea 2009;28:321-7.
6. Ray S, Khan BF, Dohlman CH, D’Amico DJ. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol 2002;120:559-66.
7. Dunlap K, Chak G, Aquavella JV, et al. Short term visual outcomes of Boston type 1 keratoprosthesis implantation. Ophthalmology 2010;117:687-92.
8. Ramos M, Kruger EF, Lashkari K. Biostatistical analysis of pseudophakic and aphakic retinal detachments. Semin Ophthalmol 2002;17:206-13.
9. Powell SK, Olson RJ. Incidence of retinal detachment after cataract surgery and neodymium: YAG laser capsulotomy. J Cataract Refract Surg 1995;21:132-5.
10. Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea 2009;28:989-96.
11. Ranta P and Kivela T. Retinal detachment in pseudophakic eyes with and without Nd:YAG laser posterior capsulotomy. Ophthalmology 1998;105:2127-33.
12. Ranta P, Tommila P, Kivela T. Retinal breaks and detachment in neodymium: YAG laser capsuolotomy: five-year incidence in a prospective cohort. J Cataract Refract Surg 2004;30:58-66.
13. Chak G, Aquavella JV. A safe Nd:YAG retroprosthetic membrane removal technique for keratoprosthesis. Cornea 2010;29:1169-72.
14. Traish AS, Chodosh J. Expanding application of the Boston type I keratoprosthesis due to advances in design and improved post-operative therapeutic strategies. Semin Ophthalmol 2010;25:239-43.
15. Yaghouti F, Nouri M, Abad JC, Power WJ, Doane MG, Dohlman CH. Keratoprosthesis: preoperative prognostic categories. Cornea 2001;20:19-23.
16. Dohlman CH, Grosskreutz CL, Chen T, et al. Shunts to divert aqueous humor to distant epithelialized cavities after keratoprosthetis surgery. J Glaucoma 2010;19:111-5.
17. Rivier D, Paula JS, Kim E, Dohlman CH, Grosskreutz CL. Glaucoma and keratoprosthesis surgery: role of adjunctive cyclophotocoagulation. J Glaucoma 1009;18:321-4.
18. Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with Boston keratoprosthesis. Cornea 2009;28:898-901.
19. Goldman DR, Hubschman JP, Aldave AJ, et al. Postoperative posterior segment complications in eyes treated with the Boston type I keratoprosthesis. Retina 2013;33:532-41.
20. Kiang L, Sippel K, Starr C, et al. Vitreoretinal surgery in the setting of permanent keratoprosthesis. Arch Ophthalmol 2012;130:487-92.
21. Srikumaran D, Munoz B, Aldave AJ, et al. Long-term outcomes of Boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology 2014;121:2159-64. | |
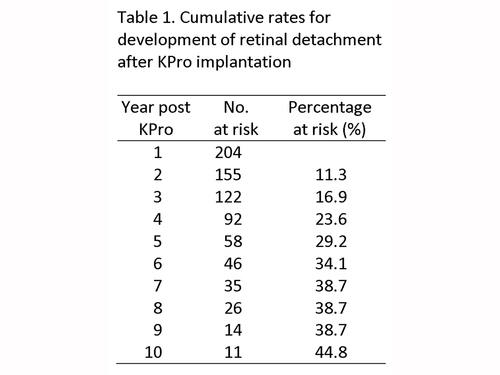
Table 1
Cumulative rates for development of retinal detachment after KPro implantation
|
|
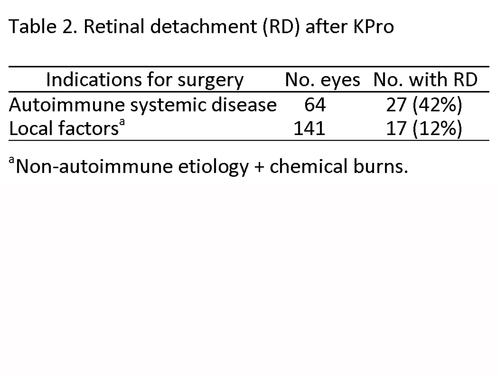
Table 2
Retinal detachment (RD) after KPro
|
|
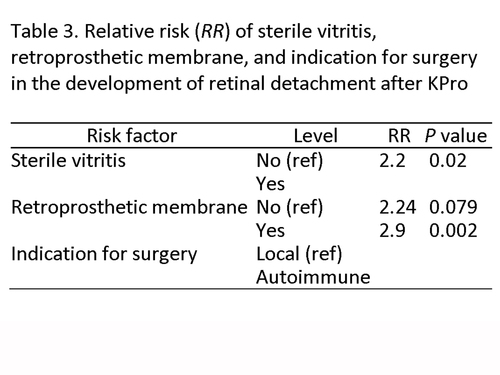
Table 3
Relative risk (RR) of sterile vitritis, retroprosthetic membrane, and indication for surgery in the development of retinal detachment after KPro
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in