|
|
 |
 |
 |
 |
|
|
A survey of preoperative blood tests in primary open-angle glaucoma patients versus cataract surgery patients
Digital Journal of Ophthalmology 2014
Volume 20, Number 2
June 30, 2014
DOI: 10.5693/djo.01.2014.02.002
|
Printer Friendly
Download PDF |



Laura Cohen, BA | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Jessica Wong, MD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Aliya Jiwani, MD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Scott Greenstein, MD | Comprehensive Ophthalmology Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Stacey Brauner, MD | Glaucoma Service and Comprehensive Ophthalmology Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Sherleen Chen | Comprehensive Ophthalmology Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Angela Turalba | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Teresa Chen, MD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Lucy Shen, MD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Douglas Rhee, MD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Janey Wiggs, MD, PhD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Jae Hee Kang, ScD | Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, Massachusetts Stephanie Loomis, MPH | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts Louis Pasquale, MD | Glaucoma Service, Massachusetts Eye and Ear and Harvard Medical School, Boston, Massachusetts; Channing Division of Network Medicine, Brigham and Women’s Hospital Boston, Massachusetts
|
|
|
| Abstract | Purpose
To investigate biomarker differences in routine preoperative blood tests performed on primary open-angle glaucoma (POAG) case and control patients presenting for anterior segment eye surgery.
Methods
POAG cases and age-related cataract surgery patients (controls) who underwent anterior segment surgery at Massachusetts Eye and Ear from January 2009 through March 2012 were identified by retrospective record review. Patients with diabetes mellitus, secondary glaucoma, and cataract due to trauma or steroid exposure were excluded. Data on demographic features, preoperative ophthalmological and medical diagnosis, blood pressure, anthropometric measures, basic metabolic panel, and complete blood count were extracted from the medical records. Univariate differences in lab values between POAG cases and controls were assessed using unpaired t tests. Multivariate logistic regression analysis was completed to determine the independent associations of biomarkers with POAG.
Results
A total of 150 cases and 150 age-related controls were included. In multivariate analysis, higher AG was inversely associated with POAG (odds ratio [OR] = 0.90; 95% confidence interval [CI], 0.80-1.00), and higher Cl− level was positively associated with POAG (OR = 1.15; 95% CI, 1.02-1.29). The lower AG in POAG patients could be explained by higher IgG levels as the available data in post hoc analysis showed a nonsignificant trend toward higher IgG in cases compared to controls (17 vs 23; 1142 ± 284 mg/dl vs 1028 ± 291 mg/dl; P = 0.22). Furthermore, in multivariable analysis, a higher red blood cell count was also associated with POAG (OR = 1.91; 95% CI, 1.11-3.28).
Conclusions
Patients with POAG presenting for anterior segment surgery had a lower AG compared to age-related cataract surgery patients. The etiology of this reduced gap is unclear but the possible contribution of IgG warrants further exploration. The etiology of higher red blood cell counts in POAG cases is unknown and deserves further exploration. | | | Introduction | The development and discovery of clinically useful biomarkers is an important area of research for primary open-angle glaucoma (POAG). A biomarker for glaucoma could identify at-risk patients for early treatment and allow for treatment response monitoring. The discovery of biomarkers associated with POAG could also provide insights into disease pathophysiology. A variety of molecules and proteins have been identified in serum, aqueous humor, and tears that are associated with POAG and represent potential disease biomarkers. They include retinal-specific antibodies, a variety of cytokines and growth factors, markers of oxidative stress, myocilin, hepcidin, and homocysteine, among others.(1-9) Because POAG is a heterogeneous disease with a multifactorial etiology, the discovery of biomarkers with clinically useful specificity and sensitivity may be challenging.
Currently, the undisputed risk factors for POAG include age, African ancestry, positive family history, and increased intraocular pressure (IOP). Other possible risk factors include myopia and decreased corneal thickness.(3,10-12) More recently, several genetic biomarkers that underlie familial predisposition have been elucidated, including allele variants in the CDKN2BAS and SIX1/SIX6 genomic regions.(13,14) Furthermore, genomic regions in GAS7 and TMCO1 have been found to harbor alleles relevant to IOP level and glaucoma pathology.(15) Studies have also shown a positive association between metabolic syndrome and high IOP and between measures of insulin resistance (which is an important component of metabolic syndrome) and POAG in some, but not all, studies.(16-21) To explore whether systemic biomarkers may be candidate POAG biomarkers, we conducted a comprehensive evaluation of all routinely obtained serum biomarkers by comparing levels in POAG and cataract patients just prior to anterior segment surgery. Any differences found might help further an overall understanding of POAG pathogenesis. | | | Materials and Methods | The Human Studies Committee at Massachusetts Eye and Ear Infirmary approved this study. The electronic medical records of consecutive POAG patients treated at the Glaucoma and Comprehensive Ophthalmology Service at the Massachusetts Eye and Ear (MEE) between January 2009 and February 2012 were reviewed retrospectively to identify patients who underwent (1) glaucoma surgery or glaucoma surgical revision without cataract surgery (n = 70), (2) glaucoma surgery combined with cataract extraction (n = 43), or (3) cataract extraction alone (n = 37). After identifying 150 cases, we identified 150 nonglaucomatous, age-related controls who underwent phacoemulsification cataract extraction with intraocular lens insertion during the study period. Cases and controls were selected for patients in the age range of 40-80 years.
There were no structural optic nerve criteria for POAG cases but mean cup-disc ratio for the operative eye was 0.78. All cases had visual field loss consistent with nerve fiber layer pathology on reliable tests. There were no IOP criteria for a diagnosis of POAG but known maximum IOP was extracted from the medical record. For secondary analyses, high-tension glaucoma (HTG) cases were defined as having a known IOP >21 mm Hg in either eye at some point before surgery; normal-tension glaucoma (NTG) cases had no known IOP >21 mm Hg at any point preoperatively. All controls had known IOP <22 mm Hg and cup-disc ratio ≥6 (the mean cup-disc ratio for controls was 0.31).
Glaucoma suspects or those with ocular hypertension (ie, no visual field loss) were excluded from the study, as were any patients meeting the following criteria: secondary glaucoma (eg, glaucoma due to exfoliation syndrome, pigment dispersion syndrome, anterior segment neovascularization, steroid use, and iridocorneal endothelial (ICE) syndrome); congenital, developmental, and juvenile glaucoma; angle-closure glaucoma or history of occludable angles requiring laser peripheral iridotomy; or patients with a diagnosis of diabetes mellitus. Finally patients with cataracts other than age-related cataracts, such as traumatic cataract or cataracts due to steroid exposure, were also excluded.
Data Collection
The following data were collected: demographic features (age, race, sex); primary open-angle glaucoma type (high or normal-tension); glaucoma-related measurements (maximum IOP, preoperative cup-disc ratio); ophthalmological and other medical diagnosis (preoperative surgical diagnosis, other ophthalmological diagnoses, medical diagnoses); preoperative physical examination measurements of blood pressure, height, and weight, laboratory studies within 6 months prior to surgery (Chem-8, a basic metabolic panel comprised of serum sodium [Na+], potassium [K+], chloride [Cl−], bicarbonate [HCO3−], blood urea nitrogen [BUN], creatinine [Cr], glucose, and calcium [Ca2+]); and complete blood count (CBC), serum albumin, and immunoglobulin G [IgG]) within 1 year before surgery and time and location of blood draw.
From this data, we calculated values for mean arterial pressure (MAP = 2/3[diastolic blood pressure] +1/3[systolic blood pressure]), body mass index (BMI, kg/m2), BUN/creatinine ratio, creatinine clearance (Cockcroft-Gault Glomerular Filtration Rate (GFR) = (140-age) * (Weight in kilograms) * (0.85 if female) / (72 * Cr)), and anion gap (AG), calculated both with potassium ([Na+] + [K+]) − ([Cl−] + [HCO3−]) and without potassium: [Na+] − ([Cl−] + [HCO3−]).
For secondary analyses to determine the source of a low AG in POAG cases, we also collected data on serum albumin and IgG within a one-year window prior to surgery, when the data was available. Furthermore, we assessed whether AG varied by POAG subtype. First, we divided POAG cases into three groups based on the type of surgery performed: cataract extraction alone; glaucoma surgery alone; and glaucoma surgery combined with cataract extraction. We reasoned that cases in the latter groups might have more poorly controlled IOP preoperatively than those in the former group. Finally we classified each case as mild, moderate, or severe, based on the most reliable visual field from the worse eye closest to the time of anterior segment surgery based on criteria recommended by the American Glaucoma Society (http://www.americanglaucomasociety.net/professionals/glaucoma_staging_codes_teaching_module/). Subsequently we assessed whether the relation between AG and POAG varied by glaucoma severity.
Statistical Analysis
SAS (v9.2, SAS Institute, Cary, NC) was used for all statistical analysis. We assessed the difference between case and control values for each Chen-8 panel member using unpaired t tests. We performed similar univariate analyses for the relation between parameters in the CBC and POAG. Subsequently, multivariate logistic regression analyses were performed to determine if parameters that were significant in univariate analysis (specifically anion gap and red blood cell count) were independently associated with POAG. Covariates included in a multivariate model of POAG where AG was the exposure of interest included age, sex, MAP, BMI, presence of other medical problems, lab test location (MEE, Massachusetts General Hospital, or other location), time of lab draw in military format and serum Ca2+ (because Ca2+ can be an unaccounted cation producing low AG). Subsequently, we also included glaucoma disease severity in the model as another covariate. In another multivariate model of POAG where serum Cl− was the exposure of interest (since higher chloride levels were the source of low AG in POAG cases), all other components of the Chem-8 panel plus the covariate list above were included as covariates. Finally, in a multivariate regression model of POAG where red blood cell count was the exposure of interest we included age, sex, MAP, BMI, presence of other medical problems, lab test location, time of lab draw, white blood cell count and platelet count as covariates. | | | Results | Cases and controls were similar in demographic features including age, sex, and race (Table 1). The majority of subjects were white, and a larger proportion of cases compared to controls were African American. A similar percentage of cases and controls were female. Approximately two-thirds of the POAG patients were HTG (97/150 [65%]) cases, and one-third were NTG cases (53/150 [35%]). MAP was slightly higher in POAG cases compared to controls.
The majority of the laboratory studies in both groups were performed at the MEE (132/150 [88%] for cases; 112/150 [75%] for controls). There were no differences between cases and controls for the white blood cell (WBC) count (6.75 ± 1.84 × 103 cells/ microliter in cases vs 6.85 ± 1.67 × 103 cells/ microliter in controls; P = 0.64) and platelet count (236.40 ± 64.24 × 103 cells/ microliter in cases vs 246.33 ± 63.02 × 103 cells/ microliter in controls; P = 0.18). However, circulating red blood cell (RBC) counts were significantly higher in cases versus controls (4.52 ± 0.51 × 106 cells/ microliter vs 4.37 ± 0.51 × 106 cells/ microliter, P = 0.01). Mean preoperative values and standard deviations for each parameter in the Chem-8 panel for POAG cases and controls are provided in Table 2. Although there was a trend toward higher random blood glucose levels in POAG cases versus controls, the difference was not statistically significant (105 ± 20 mg/dl [cases] vs 102 ± 19 mg/dl [controls]; P = 0.30). The only parameters that achieved statistical significance were AG and chloride levels. There was a lower AG (calculated with potassium) in cases than controls (9.1 ± 2.7 vs 10.2 ± 3.0; P < 0.001). This was partly driven by a higher chloride level in cases than controls (106 ± 4 vs 105 ± 4 mmol/L; P = 0.004). When the AG was calculated without potassium, statistical significance was retained (P < 0.001). This AG difference in cases and controls was not accompanied by any differences in bicarbonate levels between groups (P = 0.93; Table 2).
Post hoc analyses were completed for laboratory chemistry values that may explain lower AG values in cases including low levels of albumin, high levels of calcium, and high levels of serum paraprotein that is positively charged at physiological pH, namely IgG (22). Based on available data in the medical record (84 cases, 112 controls), albumin levels were essentially identical in cases and controls (4.2 ± 0.4 g/dl for both groups). Low albumin levels result in elevated calcium, which is also associated with a decreased AG. Calcium levels, which were available for every subject, were identical in cases and controls (9.5 ± 0.4 mg/dl in both groups). Serum IgG values were also extracted post-hoc from the medical record (n = 17 for cases, n = 23 for controls). Univariate analysis for cases vs. controls with available values revealed a non-significant trend toward higher IgG in cases (1141.9 ± 283.5 vs 1028.3 ± 290.6; P = 0.22). Multivariate analysis to assess the relationship between IgG in POAG cases was not possible due to the small sample size.
In multivariate analysis (Table 3), every unit increase in AG (accounting for serum potassium) was associated with a 10% reduced risk of POAG (odds ratio [OR] = 0.90; 95% confidence interval (CI): 0.80-1.00). In subgroup analysis, this association was driven by HTG cases (OR = 0.86; 95% CI, 0.75-0.97) because the association between AG and NTG case status (OR = 1.00; 95% CI, 0.82-1.20) was null. A separate multivariable model indicated that every 1 mmol/L Cl− was associated with a 15% increased risk of POAG (OR=1.15, 95% CI: 1.02-1.29); this association was also driven by HTG cases (OR = 1.20; 95% CI, 1.04-1.38) whereas the association between serum Cl− levels and NTG was also null (OR = 1.02; 95% CI, 0.83-1.26).
Secondary analysis also explored whether the inverse relation between AG and POAG varied by type of anterior segment surgery performed. We divided POAG cases into three groups: (1) cataract surgery alone, (2) glaucoma surgery alone, and (3) glaucoma surgery combined with cataract surgery. POAG cases requiring cataract extraction alone (n = 37) had mean AG calculated with potassium (9.5 ± 3.0) that was only slightly less than controls (10.2 ± 3.0; P = 0.2; Table 4). In contrast, POAG patients in the glaucoma surgery alone group (n = 70) or glaucoma surgery alone group added to the combined glaucoma-cataract surgery group (n = 113) had significantly smaller AGs compared to controls who underwent cataract surgery (mean AGs of 8.7 ± 2.5 [P = 0.0005] and 9.0 ± 2.8 [P = 0.0013], resp.; Table 4). Interestingly, in multivariable analysis that only considered the glaucoma surgery subgroup alone (OR = 0.86; 95% CI, 0.74-1.00; P = 0.05) or the glaucoma surgery group alone in conjunction with the combined glaucoma-cataract surgery group (OR = 0.86; 95% CI, 0.76-0.98; P = 0.02), AG remained an independent inverse predictor of POAG, suggesting that the trends noted in univariate analysis were not the result of uncontrolled confounding by age or other factors.
We also assessed whether the inverse relation between AG and POAG varied by glaucoma severity and found that 29 patients with mild stage disease based on visual field criteria had similar mean AG calculated with potassium compared to controls (10.1 ± 3.4 vs 10.2 ± 3.0 [P = 0.99]; Table 5). In contrast, 98 patients with severe disease had lower mean AG (8.8 ± 2.5) compared to POAG patients with mild disease (P = 0.017; Table 5). When disease severity was added as a covariate in multivariate analysis, AG was no longer associated with POAG (P = 0.13), suggesting that POAG patients with more severe disease were contributing to the relation between AG and POAG.
Finally, we explored whether the higher RBC count observed in POAG cases remained significant in multivariable analysis. Indeed, after controlling for age, sex, race, BMI, medical problems, lab site of analysis, time of blood draw, WBC count and platelet count, higher RBC count was associated with increased risk of POAG (OR = 1.91; 95% CI, 1.11-3.28). No case had polycythemia and no case or control had a hematological malignancy that could have skewed these results. Even when blood calcium and AG were added as covariates, the relation between higher RBC count and POAG remained essentially unchanged (OR = 1.95; 95% CI, 1.13-3.39). In subgroup analysis, this association was driven by HTG cases (OR = 1.80; 95% CI, 1.01-3.33) because the association between RBC count and NTG case status (OR = 1.80; 95% CI, 0.63-5.17) was null. Finally the association between RBC count and POAG did not vary by disease severity in both univariate and multivariable analysis (data not shown). | | | Discussion | Currently there is no systemic biomarker that can detect or monitor progression for POAG. This study attempted to identify potential biomarkers in routine lab draws, which have not been explored to date. In both univariate and multivariate analyses, we found that cases had a higher Cl− level and higher RBC counts than controls. With respect to blood chemistries, the higher Cl− contributed to a low AG in cases. Higher Cl− levels with low AG did not occur in isolation, prompting exploration of other possible causes for low AG. Secondary analyses suggested a trend toward higher paraprotein levels, which could account for cations that would serve as a source for the apparent high Cl− and low AG in POAG cases.(23) More research will be required to determine whether the biomarkers found in association with POAG in multivariable analysis are reproduced in another dataset. Furthermore, more research is needed to determine the source and significance of these biomarker differences.
Although neither cases nor controls had a serum AG out of the normal range, the POAG cases had a statistically significant one-unit lower AG. This finding was related in part to elevated chloride levels. A relatively lower AG among cases can be explained by an increase in unaccounted cations (including calcium and IgG, which is positively charged at physiological pH) or a decrease in anions (albumin), which offsets the seemingly high chloride levels.(23,24) In our data hypercalcemia and hypoalbuminemia were ruled out as the source of the lower AG in cases. Lithium can serve as another source of unaccounted-for cations but different blood lithium levels between cases seems unlikely as none of our patients were using lithium to treat any disorder.(25) Monoclonal gammopathy in multiple myeloma and monoclonal gammopathy of undetermined significance is known to cause elevated IgG and a decreased AG.(26,27) Our findings suggest that higher IgG levels in cases could be the source of the relatively low AG in cases, although more data is needed to prove this hypothesis.
The small sample size and timing of IgG values obtained in this study make it difficult to draw conclusions regarding a higher IgG levels in cases as the cause of lower AG in POAG cases. Nonetheless, investigators have speculated that a possible immune mechanism underlies glaucoma, raising the question of whether IgG is a potential biomarker for POAG. An autoimmune theory of glaucoma suggests that retinal ganglion cells stress response from ischemia and mechanical stress from high IOP leads to the production of autoantibodies. Two studies reported that patients with POAG have unique IgG antibody profiles, some of which are up regulated in aqueous humor and serum.(28,29) On further investigation, Joachim et al found unique patterns of serum IgG against ocular antigens for POAG and NTG patients compared to each other and compared to controls. The POAG group had a significant antibody profile to retinal antigens, whereas the NTG group showed a significant antibody profile to optic nerve antigens, suggesting a divergence in underlying autoimmune disease mechanisms.(30) In another study by Joachim et al., aqueous humor IgG antibody patterns were significantly different between POAG and control samples, as well as exfoliation glaucoma (EG) and control samples; yet, there was no difference between POAG and EG samples.(28) H. pylori–specific IgG levels in aqueous humor and sera of POAG patients were significantly higher in POAG patients (14.27 ± 3.86 U/ml and 69.96 ± 9.69 U/ml, resp.) compared to controls in an investigation by Kountouras et al.(31) Further, they found that the titer of H. pylori-specific IgG positively correlated with vertical cupping and disease severity.(31) Yet other investigators found no association between H. pylori infection and incidence of POAG.(32) Not all POAG patients have positive serum IgG. For example, Maruyama et al. found only 25% of POAG patients had positive serum IgG to a specific retinal antigen.(33) Recently, Tezel et al completed an immunoproteomic analysis to capture possible autoantibodies against ocular antigens and found several candidate biomarkers for glaucoma.(34) Some investigators speculate that the autoimmune nature of POAG may be specific to NTG patients, yet our analysis suggests a trend toward lower AG was driven by HTG cases.
We considered whether glaucoma treatment itself could account for the low AG in POAG. High chloride levels can occur in patients treated with systemic carbonic anhydrase inhibitors (CAIs) for glaucoma, due to renal chloride retention in response to loss of bicarbonate. However, systemic CAIs cause a non-AG metabolic acidosis.(35,36) Cases and controls had nearly identical mean serum bicarbonate of 28 mEq/L in our study (P = 0.93; Table 2); this value would be expected to be lower than 24 mEq/L if metabolic acidosis from CAI use were occurring. Similarly, the most common IOP lowering medications used in glaucoma management, topical prostaglandin analogues, are unlikely the cause of a lower AG because they have a serum half life of 45 minutes or less.(37). Furthermore topical beta blockers have no documented effect on AG.(38,39)
The most common reason for a low AG is lab error due to the use of indirect ion-selective electrodes in lab measurements, which can result in overestimation of chloride levels, especially in the presence of hypertriglyceridemia. (23,40-42) Differential lab measurement error is unlikely to be the cause for our results since most lab values for cases and controls were obtained from the same source, there was no statistical difference between source location between cases and controls, and we controlled for the lab source in multivariate analysis. There were too few subjects with available data on triglyceride levels to assess its impact on the magnitude of the AG. While hypertriglyceridemia has been shown to decrease AG with an increase in Cl−, prior work indicates that elevated triglycerides do not contribute to higher IOP, and prospective studies do not implicate dyslipidemia as a risk factor for POAG.(23,43) Thus there is no a priori reason to suspect that triglyceride levels were higher in POAG cases than controls.
Secondary analyses suggested that patients with disease that was more uncontrolled and more severe drove the inverse relation between AG and POAG; however, there were too few data to determine whether these trends were driven by higher IgG levels. Nevertheless, the trends are worthy of further study.
The retrospective nature of this study necessitated that indications for surgery stand as a surrogate for glaucoma control. The glaucoma severity scale used in this study suggested that the majority of patients had severe disease. Prospective studies exploring the source of the low AG in POAG more fully to determine whether disease severity tracks with the magnitude of the AG in POAG are worthwhile.
Interestingly we also found that POAG cases had higher RBC counts than compared to controls in multivariable analysis. No other blood parameter was significantly associated with POAG. There were trends toward higher hemoglobin levels and hematocrits in cases versus controls but no POAG patients had RBC counts above the normal range (5.80 million cells/microliter); rather, the difference seemed to relate to more patients in the control group having RBC counts that were in the lower range of normal or slightly below normal (data not shown). The difference between cases and controls is unlikely due to glaucoma treatment. Oral CAI use in cases would be expected to produce lower RBC counts, not higher ones. Unlike the association between AG and POAG, the association between RBC and POAG did not vary by disease severity. There are no prior reports of RBC counts in relation to POAG.
This study has several limitations. The findings of a lower AG and higher RBC count were not expected, which limited subsequent analyses to explain these results. With respect to the lower AG, while calcium levels were available for all subjects, albumin levels were available on 75% of cases and 56% of controls. Also IgG levels were only available on a limited number of study participants (11% of cases; 15% of controls), and there could be residual confounding by undisclosed systemic disease that prompted the availability of those tests. Other causes of decreased AG involving unmeasured cations and anions including hypermagnesemia, and bromide, lithium, or iodide intoxication could not be studied due to insufficient values in the medical record.(23) It is also unlikely that other glaucoma medicines could have caused a lower AG in cases as discussed above but this issue can be best addressed by collecting blood prospectively from newly diagnosed POAG cases and a comparable control group. A larger sample size would allow for additional trends to reach statistical significance. The associations that were not significant may reflect inadequate power to detect associations rather than a true lack of association. Another limitation is that lab values were drawn at random times. This likely contributed to our null results for blood glucose. Fasting blood glucose levels, which are standard in diabetes diagnoses, vary less (by removing glucose fluctuations associated with meals) and may provide a more accurate depiction of the differences between cases and controls. Finally, a major limitation was the exploratory nature of evaluating the panel of routine lab draws; because a large number of biomarkers were evaluated, the association with AG and with RBC count may be due to chance and should be replicated.
The differences in AG and RBC counts between POAG cases and controls described here are modest and clearly not of diagnostic value. Nonetheless, these findings may reflect important biomarkers in glaucoma that might yield insight into disease pathophysiology. | | | Acknowledgements | | This work was supported by the Harvard Glaucoma Center of Excellence, a Harvard Medical School Distinguished Scholar Award (LRP), the Arthur Ashley Foundation, and a Horizon Grant from Allergan Inc. | | | References | 1. Kang JH, Wiggs JL, Pasquale LR. A nested case control study of plasma ICAM-1, E-selectin and TNF receptor 2 levels, and incident primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2013;54:1797-804.
2. Pieragostino D, Agnifili L, Fasanella V, et al. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naive to therapy. Mol Biosyst 2013;9:1108-16.
3. Kokotas H, Kroupis C, Chiras D, et al. Biomarkers in primary open-angle glaucoma. Clin Chem Lab Med;2012;50:2107-19.
4. Tezel G, Edward DP, Wax MB. Serum autoantibodies to optic nerve head glycosaminoglycans in patients with glaucoma. Arch Ophthalmol 1999;117:917-24.
5. Huang P, Qi Y, Xu YS, Liu J, Liao D, Zhang SS, et al. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J Glaucoma 2010;19:324-30.
6. Castany M, Jordi I, Catala J, Gual A, Morales M, Gasull X, et al. Glaucoma patients present increased levels of diadenosine tetraphosphate, Ap(4)A, in the aqueous humour. Exp Eye Res 2011;92:221-6.
7. Howell KG, Vrabel AM, Chowdhury UR, Stamer WD, Fautsch MP. Myocilin levels in primary open-angle glaucoma and pseudoexfoliation glaucoma human aqueous humor. J Glaucoma 2010;19:569-75.
9. Ghanem AA, Mady SM, El awady HE, Arafa LF. Homocysteine and hydroxyproline levels in patients with primary open-angle glaucoma. Current Eye Res 2012;37:712-8.
10. Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population. Invest Ophthalmol Vis Sci 2013;54:830-5.
11. Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol 1994;112:69-73.
12. Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology 2013;120:1533-40.
13. Wiggs JL, Yaspan BL, Hauser MA, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet 2012;8:e1002654.
14. Burdon KP, Macgregor S, Hewitt AW, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet 2011;43:574-8.
15. van Koolwijk LM, Ramdas WD, Ikram MK, Jansonius NM, Pasutto F, Hysi PG, et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet 2012;8:e1002611.
16. Imai K, Hamaguchi M, Mori K, et al. Metabolic syndrome as a risk factor for high-ocular tension. Int J Obes (Lond) 2010;34:1209-17.
17. Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology 2006;113:1081-6.
18. Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology 2011;118:1318-26.
19. Tan GS, Wong TY, Fong CW, Aung T. Diabetes, metabolic abnormalities, and glaucoma. Arch Ophthalmol 2009;127:1354-61.
20. Su DH, Wong TY, Wong WL, et al. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology 2008;115:964-8.e1.
21. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53.
22. Yang H, Gurgel PV, Carbonell RG. Purification of human immunoglobulin G via Fc-specific small peptide ligand affinity chromatography. J Chromatogr A 2009;1216:910-8.
23. Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007;2:162-74.
24. Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med 2012;23:203-11.
25. Kelleher SP, Raciti A, Arbeit LA. Reduced or absent serum anion gap as a marker of severe lithium carbonate intoxication. Arch Intern Med 1986;146:1839-40.
26. van Hoeven KH, Joseph RE, Gaughan WJ, et al. The anion gap and routine serum protein measurements in monoclonal gammopathies. Clin J Am Soc Nephrol 2011;6:2814-21.
27. De Troyer A, Stolarczyk A, De Beyl DZ, Stryckmans P. Value of anion-gap determination in multiple myeloma. N Engl J Med 1977;296:858-60.
28. Joachim SC, Wuenschig D, Pfeiffer N, Grus FH. IgG antibody patterns in aqueous humor of patients with primary open angle glaucoma and pseudoexfoliation glaucoma. Mol Vis 2007;13:1573-9.
29. Rodrigues MM, Katz SI, Foidart JM, Spaeth GL. Collagen, factor VIII antigen, and immunoglobulins in the human aqueous drainage channels. Ophthalmology 1980;87:337-45.
30. Joachim SC, Pfeiffer N, Grus FH. Autoantibodies in patients with glaucoma: a comparison of IgG serum antibodies against retinal, optic nerve, and optic nerve head antigens. Graefes Arch Clin Exp Ophthalmol 2005;243:817-23.
31. Kountouras J, Mylopoulos N, Konstas AG, Zavos C, Chatzopoulos D, Boukla A. Increased levels of Helicobacter pylori IgG antibodies in aqueous humor of patients with primary open-angle and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol 2003;241:884-90.
32. Galloway PH, Warner SJ, Morshed MG, Mikelberg FS. Helicobacter pylori infection and the risk for open-angle glaucoma. Ophthalmology 2003;110:922-5.
33. Maruyama I, Ohguro H, Ikeda Y. Retinal ganglion cells recognized by serum autoantibody against gamma-enolase found in glaucoma patients. Invest Ophthalmol Vis Sci 2000;41:1657-65.
34. Tezel G, Thornton IL, Tong MG, Luo C, Yang X, Cai J, et al. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma. Invest Ophthalmol Vis Sci 2012;53:8222-31.
35. Mirza N, Marson AG, Pirmohamed M. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol 2009;68:655-61.
36. Mirza NS, Alfirevic A, Jorgensen A, Marson AG, Pirmohamed M. Metabolic acidosis with topiramate and zonisamide: an assessment of its severity and predictors. Pharmacogenet Genomics 2011;21:297-302.
37. Sjoquist B, Stjernschantz J. Ocular and systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol 2002;47 Suppl 1:S6-12.
38. Mundorf TK, Ogawa T, Naka H, Novack GD, Crockett RS. A 12-month, multicenter, randomized, double-masked, parallel-group comparison of timolol-LA once daily and timolol maleate ophthalmic solution twice daily in the treatment of adults with glaucoma or ocular hypertension. Clin Ther 2004;26:541-51.
39. Patel SS, Spencer CM. Latanoprost: a review of its pharmacological properties, clinical efficacy and tolerability in the management of primary open-angle glaucoma and ocular hypertension. Drugs Aging 1996;9:363-78.
40. Lolekha PH, Lolekha S. Value of the anion gap in clinical diagnosis and laboratory evaluation. Clin Chm 1983;29:279-83.
41. Goldstein RJ, Lichtenstein NS, Souder D. The myth of the low anion gap. JAMA 1980;243:1737-8.
42. Jurado RL, del Rio C, Nassar G, Navarette J, Pimentel JL Jr. Low anion gap. South Med J 1998;91:624-9.
43. Pasquale LR, Kang JH. Lifestyle, nutrition, and glaucoma. J Glaucoma 2009;18:423-8. | |

Table 1
Characteristics of primary open-angle glaucoma cases and cataract surgery controls
|
|
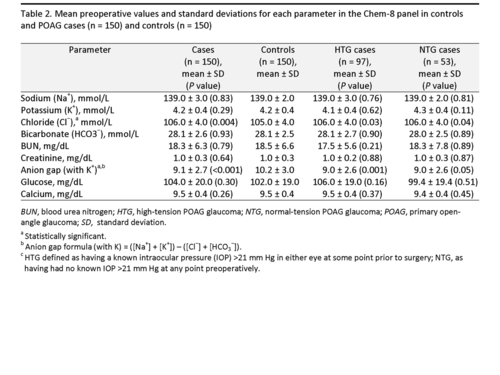
Table 2
Mean preoperative values and standard deviations for each parameter in the Chem-8 panel in controls and POAG cases (n = 150) and controls (n = 150)
|
|
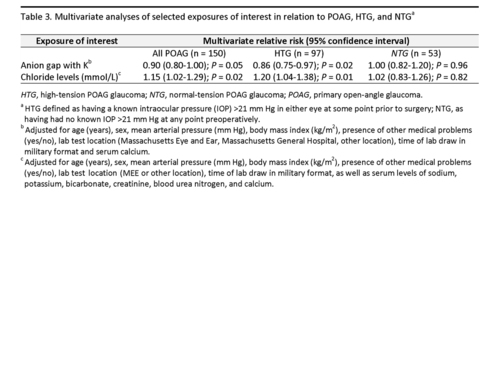
Table 3
Multivariate analyses of selected exposures of interest in relation to POAG, HTG, and NTG
|
|

Table 4
Mean anion gap calculated with potassium as a function of surgical indication
|
|
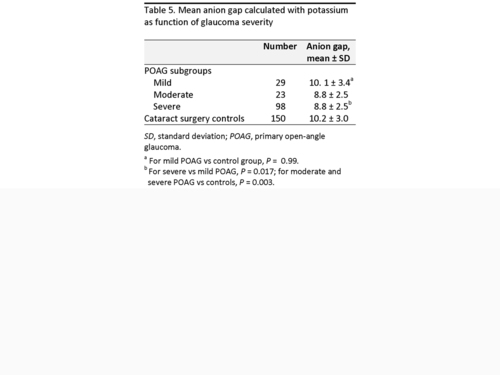
Table 5
Mean anion gap calculated with potassium as function of glaucoma severity
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in