|
|
 |
 |
 |
 |
|
|
Feasibility and efficacy of a mass switch from ranibizumab (Lucentis) to bevacizumab (Avastin) for treatment of neovascular age-related macular degeneration
Digital Journal of Ophthalmology 2015
Volume 21, Number 3
September 11, 2015
DOI: 10.5693/djo.01.2015.04.002
|
Printer Friendly
Download PDF |



Michael T. Andreoli, MD | Department of Visual Services, Harvard Vanguard Medical Associates, Boston, Massachusetts; Department of Ophthalmology and Visual Sciences, Illinois Eye and Ear Infirmary, University of Illinois at Chicago, Chicago, Illinois Michael Pinnolis, MD | Department of Visual Services, Harvard Vanguard Medical Associates, Boston, Massachusetts Troy Kieser, BS | Department of Visual Services, Harvard Vanguard Medical Associates, Boston, Massachusetts Jennifer Sun, MD | Department of Visual Services, Harvard Vanguard Medical Associates, Boston, Massachusetts; Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts; Beetham Eye Clinic, Joslin Diabetes Center, Boston, Massachusetts Christopher M. Andreoli, MD | Department of Visual Services, Harvard Vanguard Medical Associates, Boston, Massachusetts; Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts; Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts
|
|
|
| Abstract | Objective
To assess the feasibility and potential obstacles of a departmental switch from ranibizumab (Lucentis, Genentech, South San Francisco, CA) to bevacizumab (Avastin, Genentech) for the treatment of neovascular age-related macular degeneration (AMD).
Methods
A total of 154 eyes treated for wet AMD with ranibizumab or bevacizumab were examined over a 10-month period. The treatment protocol was monthly induction therapy followed by injections as needed for macular edema or subretinal fluid on optical coherence tomography, new hemorrhage or edema on examination, worsening vision, or leakage on fluorescein angiography. Central subfield thickness and pinhole vision were the main treatment outcomes. Study windows were compared using t tests and Mann-Whitney U tests. Statistical significance was defined as a P value of <0.05.
Results
The majority of patients (88%) were willing to accept a bevacizumab injection. There was no difference in frequency of injection, central subfield thickness, visual outcome, or endophthalmitis rate between the ranibizumab and bevacizumab groups. A small subset of patients (4.5%) appeared to respond more favorably to ranibizumab than bevacizumab.
Conclusion
Bevacizumab appears to be a cost-effective alternative to ranibizumab for the treatment of neovascular AMD. Patients previously treated with ranibizumab are typically willing to switch to bevacizumab. In the overwhelming majority of patients, there is no major decline in clinical status. However, select patients may respond better to ranibizumab injections.
| | | Introduction | Age-related macular degeneration (AMD) is the leading cause of blindness in individuals over 50 years of age in the United States and developed Western countries.(1,2) The neovascular form of the disease (wet AMD) is less common although more devastating when untreated. New treatments for AMD have emerged over the past decade, the most recent and efficacious of which have involved blockage of vascular endothelial growth factor (VEGF) by frequent intravitreal injection of pharmacologic agents.
Pegaptanib sodium was the first such treatment, receiving approval by the US Food and Drug Administration (FDA) in 2004 for treatment of wet AMD.(3) Ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland, and Genentech Inc, South San Francisco, CA) was later approved by the FDA for the treatment of choroidal neovascularization (CNV) in wet AMD. Ranibizumab is a recombinant, fully humanized, affinity-matured monoclonal antigen-binding antibody fragment that inhibits the binding of multiple biologically active forms of VEGF-A to their receptors.(4-6) The Minimally Classic/Occult Trial of the anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) and anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularisation in AMD (ANCHOR) studies, both of which were multicenter, randomized, double-masked trials, established the superiority of ranibizumab to prior FDA-approved treatments.(7,8) Bevacizumab (Avastin; Genentech South San Francisco, CA), another humanized monoclonal VEGF antibody, was approved by the FDA in 2004 for treatment of metastatic cancer of the colon or rectum as an intravenous infusion.(9-11) Bevacizumab has been shown to carry clinical efficacy and has been used as an off-label therapy for intravitreal injection for wet AMD.(12,13)
There has been debate over the preferred agent of choice for wet AMD, especially given bevacizumab’s off-label status for this indication and significantly lower cost per injection. A recent examination of 2008 Medicare database information revealed that bevacizumab was administered in 58% of injections to treat neovascular AMD and ranibizumab was used in 41% , revealing the former to be the more widely used drug for this indication in the Medicare population.(14)
Until recently, the evidence supporting use of bevacizumab as a treatment comparable to ranibizumab has been limited to retrospective comparisons of cases series. The Comparison of Age-related Macular Degeneration Treatments Trials (CATT) study, an NIH-sponsored multicenter, randomized controlled trial, showed similar efficacies between ranibizumab and bevacizumab at 1 year.(15,16) Furthermore, bevacizumab and ranibizumab demonstrated similar safety profiles in terms of endophthalmitis, death, venous thrombotic events, and arteriothrombotic events such as nonfatal stroke, nonfatal myocardial infarction, and vascular death.
Most studies have examined efficacies of wet AMD treatment strategies in a treatment-naive population. Stepien et al showed no significant difference in efficacy in a population switched from bevacizumab to ranibizumab.(17) More recently, 2 studies demonstrated possible tachyphylaxis to ranibizumab or bevacizumab.(18,19) However, we are not aware of any studies examining the ability to switch an established population of patients previously initiated on ranibizumab treatment to bevacizumab therapy. Additionally, we are unaware of any current or planned studies examining comparative efficacy of ranibizumab or bevacizumab on a mixed population with varying maturity anti-VEGF therapy.
The purpose of the present study was to examine the feasibility of a switch in preferred drug treatment in a population of patients with wet AMD from an FDA-approved treatment modality of ranibizumab to an off-label therapy using bevacizumab and to examine the clinical efficacy of both treatments. | | | Materials and Methods | This study was approved by the Harvard Vanguard Medical Associates Institutional Review Board and follows the requirements of the US Health Insurance Portability and Accountability Act of 1996. All patients provided informed consent.
On March 1, 2010, the investigators initiated a mass switch from ranibizumab to bevacizumab as the preferred agent for primary treatment of wet AMD. Each patient who underwent an intravitreal injection from this time forward was recommended bevacizumab as a preferred agent. The study window was considered from March 1, 2010, to December 31, 2010. The study population included chronic patients (those who had previously received ?6 injections), patients in their induction phase of treatment (1-5 prior injections), and patients who were treatment naive. Bevacizumab was recommended after an extended discussion regarding the off-label use of the drug, current literature comparing the two drugs, and cost differential of the two drugs. Both drugs remained available to the patient at their preference.
The clinical treatment strategy was not standardized; however, all three clinicians use a similar strategy of monthly injections until optical coherence tomography (OCT) showed no edema (induction) followed by pro re nata (PRN) injections. As-needed treatments were guided by presence of macular edema or subretinal fluid on OCT or new hemorrhage or edema on clinical examination, worsening vision, or leakage on fluorescein angiography. If bevacizumab therapy was not felt to be effective or if clinical outcomes were worsening as deemed by the physician, a return to ranibizumab was considered and offered.
The medical records of patients with a diagnosis of wet AMD who received an intravitreal injection of either bevacizumab or ranibizumab during the study period were retrospectively reviewed. For any patient who received an injection during this interval, all prior visits and prior injections were analyzed. The treating physicians in this group included three physicians (CA, JS, MP) at two office locations. For each included patient, demographics, visit dates, visual acuity, OCT data, and treatment type were entered into an electronic database. OCT was performed on each patient at every visit according to a standardized protocol. Central subfield thickness was recorded for all OCT scans performed on the RTVue spectral domain OCT (Optovue Inc, Fremont, CA). Visual acuity was recorded by clinic protocol using spectacle correction and additionally with pinhole improvement.
Feasibility of the mass switch was determined by the percentage of patients who were offered to switch to bevacizumab from ranibizumab or who were offered and received bevacizumab as a primary therapy at some point versus those who preferred to stay with ranibizumab. The success of the switch to bevacizumab was determined by several factors. First, those patients who were switched back to ranibizumab after at least 1 bevacizumab injection were analyzed individually. Those cases where there was evidence suggestive of the superiority of ranibizumab were reported. Evidence of superiority generally consisted of consistently better OCT central subfield thickness or visual acuity. Second, visual acuity was used as an outcome measure. Best-pinhole visual acuity was converted to logMAR for analysis. Visual acuities were analyzed over a period of time extending from 10 months prior to the study window and through the 10-month study window. Each visit during this period was assigned a drug designation corresponding to the last injection received prior to that visit by that eye. The mean vision in treatment intervals following ranibizumab injections and following bevacizumab injections were compared over the entire 20-month period in all patients. Additionally, a paired t test was performed to compare chronic patients who switched from ranibizumab to bevacizumab in the interval before and after the switch. OCT central subfield thickness was also used as an outcome measure. All visits at which an OCT scan was performed on the Optovue machine were included. Excluded were dates in which an OCT scan was not performed, scan quality was not adequate to obtain a central subfield value, or visits before the purchase of the Optovue machine at one site (September 1, 2009). The mean OCT central subfield thickness in intervals following ranibizumab injections were compared to intervals following bevacizumab injections in all patients at all visits. Additionally, a paired t test was performed to compare chronic patients who switched from ranibizumab to bevacizumab in the interval before and after the switch.
Statistical analysis was performed using GraphPad Prism software (San Diego, CA). An unpaired t test was used to compare means of continuous variables between the bevacizumab and ranibizumab groups. In a subpopulation analysis, a paired t test compared the ranibizumab and bevacizumab treatment phases in the chronic treatment patients who underwent therapy switch. Nonparametric variables were analyzed using a Mann-Whitney U test. Statistical significance was defined as a P value of <0.05. | | | Results | The study included a total of 154 eyes of 138 patients (average age, 79.4 years; range 56-104 years; 53 males [38%]). Patient demographics are summarized in Table 1. Sixteen patients (12%) had both eyes injected at some point during the study window.
Of 154 total eyes, 48 were considered naive, 29 were in the induction group, and 77 were in the chronic group (range, 6-41 injections).
A total of 593 injections of bevacizumab were administered during the study window. Two patients who entered the study died during the study window. Two patients included in the study population developed endophthalmitis. One occurred during the study window after subsequent switch back to ranibizumab following initial switch to bevacizumab for several injections. The other case of endophthalmitis occurred in 2009, prior to the study window following a ranibizumab injection. This patient continued to receive injections after complete resolution of the infection and inflammation. One patient had alternating bevacizumab and ranibizumab injections every 2 weeks due to perceived lack of sufficient efficacy with either drug monthly.
Table 2 outlines the treatment switch for all of the patients. Of the eyes in the study window, 134 received a bevacizumab injection at least once (86%). Twenty of the eyes in the study window never received a bevacizumab injection. When broken down by treatment group, 48 eyes were in the treatment-naive group and all were started on bevacizumab as an initial therapy. Of the 29 patients in the induction group, 21 (72%) switched to bevacizumab and 8 remained with ranibizumab. Of the 77 eyes in the chronic group, 64 (83%) switched to bevacizumab and 13 remained with ranibizumab.
Fourteen eyes that had received at least 1 bevacizumab injection later received at least 1 ranibizumab injection during the study interval. Of these, 6 eyes were felt to show evidence suggestive of a better clinical response to ranibizumab, whereas the remaining 8 did not show an improvement with switch back to ranibizumab.
Another measure of relative effectiveness of the two therapies was the number of injections per year (see Table 3). In the entire population, including all patients prior to therapy switch and those patients who stayed with ranibizumab, the mean number of ranibizumab injections per year was 6.9. The mean number of bevacizumab injections per year in those that switched therapies was 7.6 (P = 0.1686). When using the same metric in only those in the chronic group that switched therapies, the mean number of injections per year was 7.0 for ranibizumab and 6.9 for bevacizumab (P = 0.8377).
OCT central subfield thickness at each visit during a period from 10 months prior to the start of the study until the end of the study was analyzed (May 1, 2009, to December 31, 2010). In the full population, the mean OCT central subfield thickness for those visits following a bevacizumab injection was 266.5 and visits following ranibizumab injections was 264.8 (P = 0.6240; Table 3). For the chronic patients who underwent therapy switch alone, the mean OCT central subfield thickness following a bevacizumab injection was 265.4 and for ranibizumab injections was 265.8 (P = 0.7289).
The best-corrected pinhole visual acuity was converted to logMAR for analysis and averaging and reported as logMAR and Snellen equivalent. For the entire cohort, the mean visual acuity during a ranibizumab treatment window was 0.46 logMAR (20/57 Snellen equivalent) and during a bevacizumab treatment window was 0.48 logMAR (20/60 Snellen equivalent; P = 0.2256). For the chronic patients who underwent therapy switch alone, the mean visual acuity during a ranibizumab treatment window was 0.42 (20/53 Snellen equivalent) and during a bevacizumab treatment window was 0.46 (20/58 Snellen equivalent; P = 0.1232). | | | Discussion | The main goal of the study was to examine the ability to switch a large population of wet AMD patients from ranibizumab (the gold standard FDA-approved treatment modality at the time of the study) to bevacizumab (an established, more widely used, off-label treatment option). The results of this study confirm that it is possible to switch a mixed population of chronic and treatment-naïve patients with wet AMD from ranibizumab to bevacizumab. Following discussion regarding FDA approval status, price, and status of current research, 88% of patients were willing to receive a bevacizumab injection. When examining this by population subtype, patients in the treatment-naïve group were all willing to begin treatment with bevacizumab following a discussion of both options, with a lower rate in the chronic group and even lower in the induction group.
Using a nonstandardized induction until dry then PRN injection strategy, there was no obvious difference in outcomes between the bevacizumab and ranibizumab groups. OCT central subfield thickness was nearly identical when comparing patients during ranibizumab injection windows to the bevacizumab injection windows. The average pinhole visual acuity was also similar between the groups. The time between injections was slightly shorter for bevacizumab than ranibizumab but was not statistically significant. There has been prior suggestion from animal studies(20,21) and a retrospective case series(22) that bevacizumab may have a longer effective half life; however, this was not confirmed by clinical efficacy in either the CATT study(15,16) or our data.
The unique features of our practice may have implications on the willingness of patients to accept treatment changes. Although we are not currently a health maintenance organization (HMO), our physician organization had its roots in such a model. Many of our patients have remained with our physician group since this organizational change. Also, a large portion of our wet AMD patients have a Medicare-preferred insurance plan, which carries a capitated risk strategy. Such a population may be more experienced with, and willing to accept, limited treatment options based on factors such as cost effectiveness.
Although our population was highly accepting of a switch to bevacizumab, this switch predated the release of the CATT study results.(15,16) With rigorous proof of efficacy and safety, one would assume that a population would be even more accepting of such a change.
Additionally, although the nature of our payer mix incentivizes our organization to preferentially use a less expensive treatment modality, the decision to undergo a mass switch was made entirely by the clinicians, with no suggestion by the organization. The clinicians sought approval to make the switch clinically and were required by the organization to measure and report certain safety measures. Additionally, none of the clinicians/investigators or the department of visual services benefited financially directly from the cost savings involved in using a less expensive therapy. The organization is a nonprofit, multispecialty physician group. The cost savings seen by the organization, however, were not distributed to the department of visual services or the investigators. Also, the difference in cost of the therapies was discussed with each patient. Continual review of patient billing records was performed to ensure that no patient was negatively affected financially from the decision to switch therapies.
The cost of bevacizumab at our organization is $22 per injection. Ranibizumab costs $1,950 per injection. At our organization, the decreased cost represents a savings to a combination of Medicare, various insurance payers, and our institution. With a savings of $1,928 per injection for a total of 593 injections during this study, a total savings of $1,143,304 was seen. In this small study, at one organization, this highlights the incredible savings possible to the health care system by using bevacizumab rather than ranibizumab.
Although the present study was not a rigorous randomized controlled trial, several factors were analyzed to investigate relative efficacy and used as a marker of relative “safety” in switching from ranibizumab to bevacizumab. Of the 134 eyes that received as least 1 bevacizumab injection, a high percentage remained with that therapy. Fourteen eyes were later switched to ranibizumab at some point during the 10-month study window. This switch was made at investigator or patient discretion. Of those who switched back to ranibizumab, 8 either switched back to bevacizumab or were felt to have no difference in treatments. The remaining 6 patients were felt by the clinicians and verified by retrospective review by the authors to have had a suggestion of a superior treatment response to ranibizumab. Although the CATT study(15,16) shows equivalent efficacy in vision outcomes at 1 year, there may be some patients in whom ranibizumab therapy provides superior results. Alternatively, observed differences in effectiveness may be due to tachyphylaxis to bevacizumab or ranibizumab. The present study was not designed or powered to directly compare the efficacy of these two therapies.
The present study is limited by its overall design and retrospective nature. Our analysis of efficacy fails to meet the hallmarks of a prospective, randomized trial. Nevertheless, OCT measurements do provide a well-accepted, objective measurement. The visual acuity measurements were not recorded using ETDRS charts or with standardized refractions. The lack of randomization introduces significant bias in the efficacy results. Although all patients were recommended to switch therapies, this was not required. It is possible that those patients doing more poorly may have been more readily predisposed to try a new therapy or conversely more hesitant to switch to a nonapproved therapy.
In summary, a mass switch from ranibizumab to bevacizumab is possible with high rate of acceptance in a large population of mixed treatment maturity. Overall, a switch to bevacizumab resulted in similar clinical outcomes; however, there a was small percentage of patients who seemed to respond better clinically to ranibizumab therapy. We recommend consideration of use of bevacizumab therapy as a primary therapy with an aim to reduce health care costs, while considering a trial of ranibizumab therapy in those patients with suboptimal response.
Author Contributions
Design and conduct of study: CMA, MP, TK, MTA, JS; data collection: CMA, TK, MTA; management: CMA, MP, TK, MTA, JS; analysis: CMA, MTA; interpretation of data: CMA, MP, MTA, JS; preparation, review and approval of manuscript: CMA, MP, TK, MTA, JS. | | | References | 1. Friedman DS, O’Colmain BJ, Munoz B, et al; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564-72.
2. Congdon N, O’Colmain B, Klaver CC, et al; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122:477-85.
3. Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-16.
4. Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol 1999;293:865-81.
5. Muller YA, Chen Y, Christinger HW, et al. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure 1998;6:1153-67.
6. Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997;57:4593-9.
7. Rosenfeld PJ, Brown DM, Heier JS, et al; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31.
8. Brown DM, Kaiser PK, Michels M, et al; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-44.
9. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21:60-5.
10. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
11. Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427-34.
12. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005;36:331-5.
13. Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006;113:363-72.e5.
14. Brechner RJ, Rosenfeld PJ, Babish JD, Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. Am J Ophthalmol 2011;151:887-95.e1.
15. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908.
16. Martin DF, Maguire MG, Fine SL, et al; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98.
17. Stepien KE, Rosenfeld PJ, Puliafito CA, et al. Comparison of intravitreal bevacizumab followed by ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2009;29:1067-73.
18. Eghoj MS, Sorensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol 2012;96:21-3.
19. Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 2012;96:14-20.
20. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007;114:2179-82.
21. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114:855-9.
22. Fong DS, Custis P, Howes J, Hsu JW. Intravitreal bevacizumab and ranibizumab for age-related macular degeneration a multicenter, retrospective study. Ophthalmology 2010;117:298-302. | |
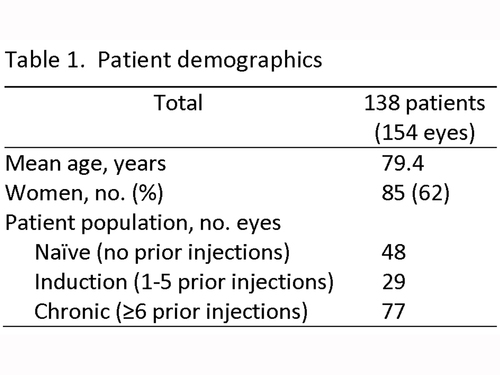
Table 1
Patient demographics
|
|
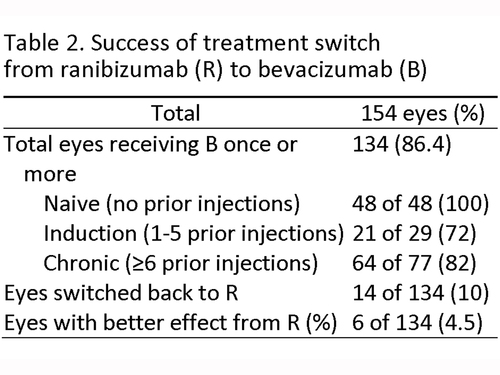
Table 2
Success of treatment switch
from ranibizumab (R) to bevacizumab (B)
|
|

Table 3
Efficacy of treatment with ranibizumab (L) and bevacizumab (B)
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in