|
|
 |
 |
 |
 |
|
|
Effects of topical travoprost 0.004% on intraocular pressure and corneal biomechanical properties in an animal model
Digital Journal of Ophthalmology 2016
Volume 22, Number 1
February 11, 2016
DOI: 10.5693/djo.01.2015.03.001
|
Printer Friendly
Download PDF |


 Gabriel Lazcano-Gomez, MD
Gabriel Lazcano-Gomez, MD | Glaucoma Department, Asociación para Evitar la Ceguera en Mexico I.A.P., San Lucas Coyoacan, Mexico DF David Ancona-Lezama, MD | Retina Department, Asociación para Evitar la Ceguera en Mexico I.A.P., San Lucas Coyoacan, Mexico DF Felix Gil-Carrasco, MD | Asociación para Evitar la Ceguera en Mexico I.A.P., San Lucas Coyoacan, Mexico DF Jesus Jimenez-Roman, MD | Glaucoma Department, Asociación para Evitar la Ceguera en Mexico I.A.P., San Lucas Coyoacan, Mexico DF
|
|
|
| Abstract | Purpose
To determine whether topical application of travoprost 0.004% induces changes in corneal biomechanical properties affecting intraocular pressure (IOP) values in rabbits.
Methods
Both eyes of 10 New Zealand rabbits were measured 3 times with the Ocular Response Analyzer (ORA) before treatment. Each measurement included corneal hysteresis (CH), corneal resistance factor (CRF), corneal-corrected IOP (IOPcc), and Goldmann equivalent IOP (IOPg). A drop of travoprost 0.004% was applied once daily in right eyes for 3 months; left eyes received no treatments. After 3 months of treatment both eyes of all rabbits were again measured 3 times. After complete keratectomy of both eyes, tissues prepared with hematoxylin-eosin stain were analyzed by means of light microscopy.
Results
The mean pre- and post-treatment IOPg, respectively, for right eyes was 9.92 ± 5.64 mm Hg and 7.62 ± 2.99 mm Hg (P = 0.027); IOPcc, 19.81 ± 5.25 mm Hg and 17.79 ± 4.09 mm Hg (P = 0.063); CRF, 1.65 ± 1.63 mm Hg and 2.18 ± 2.50 mm Hg (P = 0.266); and CH, 2.79 ± 1.74 mm Hg and 2.64 ± 2.08 mm Hg (P = 0.72). Mean post-treatment right and left eye IOPg values were, respectively, 7.62 ± 2.99 and 10.30 ± 4.40 (P = 0.002); IOPcc, 17.79 ± 4.09 mm Hg and 20.37 ± 4.32 mm Hg (P = 0.009); CRF, 1.65 ± 1.63 mm Hg and 2.17 ± 2.47 mm Hg (P = 0.274); and CH, 2.79 ± 1.74 mm Hg and 2.54 ± 2.08 mm Hg (P = 0.575). No difference in CH and CRF was observed between treated and untreated eyes.
Conclusions
Post-treatment reduction of IOP in treated eyes was a direct hypotensive effect of travoprost 0.004% and was not affected by changes in corneal biomechanical properties (CH and CRF), resulting in real lower IOP values. | | | Introduction | Prostaglandin analogues (PGAs) have become the first-line therapy for patients with glaucoma.(1) PGAs promote aqueous humor outflow by the uveoscleral pathway and, to a lesser extent, through the trabecular meshwork and Schlemm’s canal, which are part of the conventional pathway.(2) This effect is due to connective tissue remodeling by active metalloproteinases in the ciliary body and trabecular meshwork.(3) Recent studies suggest that corneal biomechanical properties may provide important information about glaucoma progression, such as increased deformation of the optic nerve surface.(4) Corneal hysteresis (CH) is considered to be a direct measurement of corneal biomechanical properties, with central corneal thickness (CCT) being one of the factors directly related to it.(5) Corneal resistance factor (CRF) is also believed to be useful in predicting glaucoma progression and may be correlated with CCT.(6,7)
Recently Tsikripis et al prospectively analyzed the effect of latanoprost and latanoprost/timolol combination on corneal biomechanical properties and CCT and concluded that low CH and a thin cornea may lead to underestimation of intraocular pressure (IOP); they suggested that the Ocular Response Analyzer (ORA; Reichert Ophthalmic Instruments, Depew, NY) be used in combination with Goldmann applanation tonometry (GAT) to obtain more accurate and reliable IOP values.(8) Multiple articles have analyzed the effects on corneal biomechanics of specific PGAs, including latanoprost or bimatoprost.(9) Agarwal et al retrospectively compared the relationship between CH and the magnitude of IOP reduction with PGA, and they found that baseline CH is independently associated with the magnitude of IOP reduction with PGA therapy.(10)
Experimental studies have demonstrated that PGAs alter corneal collagen structure and affect CCT.(10) These changes imply that results of GAT could be affected by PGA treatment.(11-13) In a study of 136 human eyes, Schlote et al found that topical therapy with travoprost was accompanied by a significant reduction of CCT within 1 year of treatment.(12) In a study with F2? prostaglandin, Viestenz et al found that CCT in PGA-treated eyes decreased significantly, suggesting that these changes might be attributed to effects of F2? PGA on the extracellular matrix of corneal stroma via upregulation of matrix metalloproteinases.(13)
It is not known whether PGAs induce changes in corneal biomechanical structure or, subsequently, affect GAT measures, which assume that there is minimal variation due to the biomechanical properties of the cornea and to CCT. However, in clinical practice, corneal thinning under local effect of PGA treatment could result in underestimation of IOP levels as measured by applanation tonometry.(14,15) Bergonzi et al used A-scan laser confocal microscopy to analyze 52 eyes that had only been on PGA therapy for 3 years and concluded that PGA therapy increased the keratocyte density in each layer of corneal stroma but more significantly in the anterior stroma than in the midstroma or the posterior stroma.(16)
The increased keratocyte density might be the result of diminished extracellular matrix, possibly owing to the activation of matrix metalloproteinases and inhibition of their tissue inhibitors.(17) The current interest in improving methods of IOP measurement has led to study of the influence of corneal biomechanical properties on the development of glaucoma.
The Ocular Response Analyzer performs measurements of corneal properties such as corneal hysteresis (CH) and corneal resistance factor (CRF) and incorporates both Goldmann-correlated IOP (IOPg) and corneal-corrected IOP (IOPcc), a measurement that is minimally influenced by corneal properties.(17) CH is defined as the difference between the air-jet pressure at inward and outward applanation and is considered to be a measure of corneal viscous damping and thus the cornea’s resistance to deformation.(18-20) Lower CH values have been linked with progression of visual fields in primary open-angle glaucoma (POAG); lower CH values, with worse visual field damage in patients with asymmetric POAG.(11) The purpose of the present study was to determine whether topical application of travoprost 0.004% induces changes in corneal structure and biomechanical properties affecting intraocular pressure measures in rabbits. | | | Materials and Methods | The prospective longitudinal and experimental study was reviewed and approved by the local internal review board of the Asociación para Evitar la Ceguera en Mexico I.A.P. Dr. Luis Sanchez Bulnes and conforms to all guidelines of the “Statement for the Use of Animals in Ophthalmic and Visual Research” (Association for Research in Vision and Ophthalmology, http://www.arvo.org/about_arvo/policies/statement_for_the_use_of_animals_in_ophthalmic_and_visual_research/#investigators). The study was sponsored by the Asociación para Evitar la Ceguera en Mexico I.A.P. Research funding was also provided by the Glaucoma Department of the same institution.
Ten 6-month-old female New Zealand rabbits were utilized. Each rabbit weighed approximately 3 kg. All the rabbits were anesthetized intravenously with pentobarbital (25 mg/kg dose). A drop of topical tetracaine 0.5% was applied to both eyes, and each eye was measured 3 times with the ORA when the rabbit was completely anesthetized (approximately 5 minutes after pentobarbital was administered).
Baseline and post-treatment measurements were obtained in the same fashion. To obtain measurements, a small eyelid speculum was used to keep the rabbit’s eyes open. Each rabbit was held gently by its ears, we centered each eye in front of the automatic “air puff” system at approximately 4 cm until an automatic measurement was performed. This procedure was repeated 3 times for each eye by the same tester. Mean values of CH, CRF, IOPg, and IOPcc were calculated using the 3 measurements for each eye.
One drop of travoprost 0.004% was applied daily at 9:00 a.m, to the right eye of all rabbits for 3 consecutive months. After 3 months of treatment both eyes were again measured 3 times each. After measuring corneal properties and IOP with ORA, both eyes were enucleated using a scalpel and corneal scissors. A complete keratectomy in both eyes was performed. Before enucleation of both eyes, 50 mg/kg of intravenous pentobarbital (for a total dose of 75 mg/kg) was administered to each rabbit to achieve euthanasia. Corneal tissue was fixed in formaldehyde to be analyzed by light microscopy. An experienced ophthalmic pathologist compared the 20 corneas using Hematoxylin-eosin stain. Data on each slide depicting treated or control group was not masked for the pathologist. Any histological difference between eyes was reported. ORA data was collected in a spreadsheet and values were analyzed using a one-sided t test for paired samples. A P value of <0.05% was considered statistically significant. | | | Results | | Pre- and post-treatment measurements (IOPg, IOPcc, CRF and CH) of both eyes are provided in Table 1. We found no histological differences in endothelium, stromal, or epithelial cells between treated and untreated eyes. Corneal epithelial hydropic degeneration was observed in all eyes (Figure 1). | |

Figure 1
Light microscopy of corneal tissue (hematoxilin-eosin, original magnification ×40). A, Travoprost-treated right eye. B, Untreated left eye. Evidence of hydropic degeneration of the corneal epithelium with no difference in the arrangement or structure of stromal collagen fibers in both eyes is illustrated with the arrows.
|
|
| Discussion | The present study shows that PGAs decrease IOP by increasing uveoescleral outflow of aqueous humor and not by changing the biomechanical properties of the cornea, which could result in falsely lower values of IOP with GAT. IOP values decreased only in the right eyes of rabbits after treatment with travoprost 0.004%. IOPg was the only value that had a statistically significant decrease. Although not statistically significant, a decrease in IOPcc values was observed. When comparing post-treatment values between right and left eyes, an important difference was observed in IOPg and IOPcc values; this was due to the hypotensive effect of travoprost 0.004% on the right eyes.
Tsikripis et al observed that the magnitude of IOP reduction with PGA was significantly associated with CH and that there was a negative statistically significant correlation, particularly in the first semester.(8) In our study CFR and CH values were not affected after travoprost treatment; this became evident when comparing pre- and post-treatment values of right eyes and post-treatment values of right and left eyes. This could be explained by the short duration of our 3-month experiment versus the changes found by Tsikripis et al in the first 6 months. In our study CRF did not significantly change in both groups. These results correlate with those of Tsikripis et al.
Although GAT remains the gold standard for IOP control, ORA has been shown to be a reliable instrument for IOP monitoring, not only by analyzing IOP values but also by measuring corneal biomechanical properties that could be affected by corneoescleral pathologies but not by PGAs, as demonstrated by this study.
PGAs have been shown to produce changes in CCT by diminishing stromal keratocytes. This effect is secondary to the activation of matrix metalloproteinases and degradation of extracellular matrix. The present study did not analyze CCT, although CH is directly related to CCT.
There are several limitations in our study. The small sample number and the use of pentobarbital as an anesthetic in rabbits are the most important. It is well known that anesthetics may increase intraocular pressure, thus affecting the IOP values in this study. We observed that the only parameter that was significantly affected by PGA treatment was IOPg; the biomechanical properties of the cornea (CH, CRH) were not affected. The reduction in the IOP in right eyes was due to a direct hypotensive effect of travoprost 0.004% and was not affected by changes in corneal biomechanical properties.
Although this study was performed using an animal model and the population size is small, reliable results about the behavior of the pressure and corneal properties in animals were observed. More studies in animals or humans are needed to corroborate our findings. | | | Acknowledgements | Travatan 0.004% was donated by Alcon Pharmaceutics Mexico.
Funding support
Association to Prevent Blindness in Mexico.
| | | References | 1. Denis P, Baudouin C. First-line latanoprost therapy in ocular hypertension or open angle glaucoma patients: a 3-month efficacy analysis stratified by initial intraocular pressure. BMC Ophthalmol 2010; 10:4.
2. Toris C. Aqueous humor dynamics. In: Choplin N, Lundy D, eds. Atlas of Glaucoma. London: Informa; 2007:13-25.
3. Shacknow P, Samples J. Medications used to treat glaucoma. In: Shacknow P, Samples J, eds. The Glaucoma Book. London: Springer; 2010:583-628.
4. Wells AP, Garway-Heath DF, Poostchi A, Wong T, Chan KC, Sachdev N. Corneal hysteresis but not corneal thickness correlated with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci 2008;49:3262-8.
5. Shih CY, Graff Zivin JS, Trokel SL, Tsai JC. Clinical significance of central corneal thickness in the management of glaucoma. Arch Ophthalmol 2004;122:1270-5.
6. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:714-20.
7. Brandt JD, Beiser JA, Gordon MO, Kass MA. Central corneal thickness and measured IOP response to topical ocular hypotensive medication in the Ocular Hypertension Treatment Study. Am J Ophthalmol 2004;138:717-22.
8. Tsikripis P, Papaconstantinou D, Koutsandrea, Apostolopoulos M, Georgalas I. The effect of prostaglandin analogs on the biomechanical properties and central thickness of the cornea of patients with open-angle glaucoma: a 3-year study on 108 eyes. Drug Des Devel Ther 2013;7:1149-56.
9. Noecker RS, Dirk MS, Choplin NT, Bernstein P, Batoosingh AL, Whitcup SM; Bimatoprost/Latanoprost Study Group. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol 2003;135:55-62.
10. Agarwal DR, Ehrlich JR, Shimmyo M, Radcliffe NM. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. Br J Ophthalmol 2012;96:254-7.
11. Congdon N, Broman AT. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophtalmol 2006;141:868-75.
12. Schlote T, Tzamalis A, Kynigopoulos M. Central corneal thickness during treatment with travoprost 0.004% in glaucoma patients. J Ocul Pharmacol Ther 2009;25:49-159 62.
13. Viestenz A, Martus P. Impact of prostaglandin-F (2alpha)-analogues and carbonic anhydrase inhibitors on central corneal thickness—a cross-sectional study on 403 eyes. Klin Monbl Augenheilkd 2004;221:753-6.
14. Brigatti L, Maguluri S. Reproducibility of self-measured intraocular pressure with the phosphene tonometer in patients with ocular hypertension and early to advanced glaucoma. J Glaucoma 2005;14:36-9.
15. Danesh-Meyer HV, Niederer R, Gaskin BJ, Gamble G. Comparison of the proview pressure phosphene tonometer performed by the patient and examiner with the Goldmann applanation tonometer. Clin Experiement Ophthalmol 2004;32:29-32.
16. Bergonzi C, Giani A, Blini M, Marchi S, Luccarelli S, Staurenghi G. Evaluation of prostaglandin analogue effects on corneal keratocyte density using scanning laser confocal microscopy. J Glaucoma 2010;919:617-21.
17. Goebels SC, Seitz B, Langenbucher A. Precision of ocular response analyzer. Curr Eye Res 2012;37:689-93.
18. Congdon NG, Broman AT, Bandeen-Roche K. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 2006;141:868-75.
19. Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg 2005;31:156-62.
20. Touboul D, Roberts C, Kérautret J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg 2008;34:616-22. | |
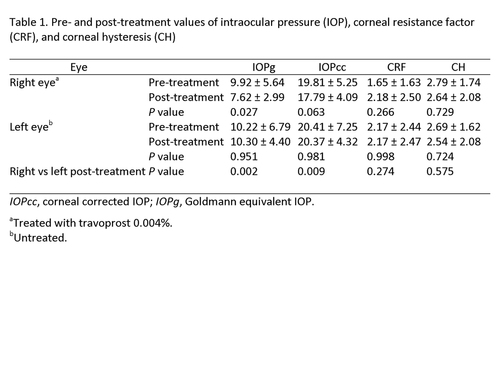
Table 1
|
|
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in