|
|
 |
 |
 |
 |
|
|
Combined intravitreal ranibizumab and verteporfin photodynamic therapy versus ranibizumab alone for the treatment of age-related macular degeneration
Digital Journal of Ophthalmology 2011
Volume 17, Number 3
September 29, 2011
DOI: 10.5693/djo.01.2011.05.003
|
Printer Friendly
Download PDF |



The Luce Study Group | Department of Ophthalmology, University of Rome Rosalia Giustolisi, MD | Policlinico Umberto I Rome Nicoletta Fantozzi, MD | Policlinico Umberto I Rome Mariateresa Staltari, MD | Policlinico Umberto I Rome Jessica Marchiori, MD | Policlinico Umberto I Rome Olga Mastrangelo, MD | Policlinico Umberto I Rome Roberta Marcucci, MD | Policlinico Umberto I Rome Federica Mirra, MD | Policlinico Umberto I Rome Paola Mazzotta, MD | Policlinico Umberto I Rome Corrado Balacco Gabrieli, MD | Policlinico Umberto I Rome
|
|
|
| Abstract | Purpose
To compare same-day combined therapy of photodynamic therapy with verteporfin (PDT-V) and intravitreal ranibizumab versus monotherapy with ranibizumab for the treatment of choroidal neovascularization.
Methods
In this prospective study, the total number of eyes were randomized into two groups: in the first, treatment consisted of a combined therapy of PDT-V and ranibizumab 0.5 mg on the same day; in the second, ranibizumab 0.5 mg in 3 monthly injections. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) on optical coherence tomography (OCT) were recorded before and 6 months after treatment.
Results
A total of 47 eyes of 47 subjects were enrolled in the study. In the combined-therapy group (group 1), the mean baseline BCVA ± standard deviation (SD) was 32.65 ± 11.09 letters (Snellen equivalent, 20/59); in the ranibizumab-alone group (group 2), 29.13 ± 9.03 letters (20/70). At 6 months’ follow-up, in group 1 the mean baseline BCVA was 39.06 ± 10.12 letters (20/42); in group 2, 33.87 ± 12.06 letters (20/57). Improvement was significant in both group 1 (P = 0.03) and group 2 (P = 0.002). In group 1, the mean CMT at baseline ± SD was 315 ± 95.49 µm; in group 2, 306.33 ± 71.61 µm. At 6 months’ follow-up, in group 1 it was 202 ± 52.02 µm; in group 2, 226 ± 65.58 µm. Reduction was significant in both group 1 (P = 0.0007) and group 2 (P = 0.00001). After 6-months, the rate of retreated eyes was 29.4% in group 1 and 43.3% in group 2. The need for retreatment did not depend on the treatment protocol (P = 0.34).
Conclusions
From a functional and anatomic point of view, the two treatments showed equivalent efficacy, with fewer retreatments in group 1. No serious adverse events, such as retinal detachment, endophthalmitis, or ocular hypertension occurred in either group. | | | Introduction | Age-related macular degeneration (AMD) is the leading cause of blindness and visual disability in patients aged 60 years and older in Europe and North America. Worldwide, after cataract and glaucoma, AMD is the third leading cause of blindness, contributing to 8.7% of all legal blindness.(1) Although the majority of patients with AMD have the non-neovascular form, characterized by drusen and atrophic changes in the retinal pigment epithelium, up to 90% of severe vision loss caused by AMD is attributable to the neovascular (exudative) form of the condition, which is characterized by choroidal neovascularization (CNV).(2) With CNV, new vessels invade the retina and sub-retinal space and eventually bleed and leak serous fluid due to their altered permeability. The blood and serous fluid causes alterations in the macular, distorting images and causing loss of visual acuity. Although the natural course of CNV secondary to AMD is highly variable, the long-term prognosis is poor.(3)
Several therapeutic options are available. In 2000 photodynamic therapy with verteporfin (PDT-V) was approved by the United States’ Food and Drug Administration for the treatment of subfoveal CNV due to AMD.(4) Recently, clinical investigations have studied a new class of drugs for subfoveal CNV: vascular endothelial growth factor (VEGF) selective inhibitors, administered via intravitreal injection.(5,6) More recently, clinicians have been offering a combined treatment, consisting of PDT followed by an intravitreal injection of an anti-VEGF drug within 24 hours. The combination of two treatments should have better results in preventing the growth of new vessels.(7-10) The purpose of our study is to compare combined therapy of PDT-V and intravitreal ranibizumab versus monotherapy with ranibizumab. | | | Materials and Methods | This study was conducted in compliance with the Ethics Committee of the Department of Ophthalmology of the University of Rome "La Sapienza" and in accordance with all state laws in Italy. Prior to determination of full eligibility for enrollment, all patients provided written, informed consent.
Our open-label, single-center, randomized controlled trial was designed to compare the efficacy of combined therapy, defined as intravitreal ranibizumab 0.5 mg and PDT administered on the same day, versus a group treated with ranibizumab-alone in eyes with primary classic CNV secondary to AMD. Inclusion criteria were a best-corrected visual acuity (BCVA) letter score equal or better than 10 letters (Snellen equivalent 20/200), classic subfoveal CNV lesions due to AMD, greatest linear dimension (GLD) of the entire lesion ¡Ü5400 ¦Ìm, at least 55 years of age, and active CNV secondary to AMD with evidence of leakage on fluorescein angiography. Exclusion criteria were previous treatment with bevacizumab or pegaptanib. Patients previously treated with PDT were excluded. Patients were also excluded if they had uncontrolled diabetes, a history of coagulation disorders, cerebrovascular accidents, pulmonary embolism, deep vein thrombosis, uncontrolled systemic hypertension, chronic renal failure, myocardial infarction within the previous 6 months, major surgery within the previous 6 weeks, ocular diseases that could affect visual acuity (eg, glaucoma, angioid streaks, trauma, choroiditis, hereditary diseases [even to fellow untreated eyes], aphakia), or previous vitreoretinal surgery. Patients were randomized into two groups with a 1:2 ratio: group 1 patients received the combined therapy; group 2 patients, monotherapy with ranibizumab.
All patients underwent full ophthalmologic examination at baseline and during the follow-up period. BCVA of both eyes was measured by an orthoptist masked to the study using a modified Early Treatment of Diabetic Retinopathy Study (ETDRS) chart at 3 meters distance. Visual acuity improvement was defined as a gain of at least 3 ETDRS lines (15 letters), stability as a change of less than 3 ETDRS lines, and deterioration as a loss of at least 3 ETDRS lines. Central macular thickness (CMT) examinations were performed using a STRATUS OCT (V4.01 Zeiss Meditec, Dublin, CA). CMT was assessed using the high-resolution Radial Lines protocol and the Retinal Thickness Map analysis program. AMD neovascular lesions were defined by intraretinal hemorrhages and edema. From the baseline thickness, a decrease in CMT of ¡Ý10% was defined as a reduction and an increase ¡Ü10% was defined as an increase. Fluorescein angiography was performed by three trained unmasked photographers using a HRA2 FA (Heidelberg Engineering). The presence of CNV leakage was evaluated in the late (3-5 min) versus the early (first 1-2 min) phase. The leakage was compared between the times before and after treatment and was described as absent (CNV closure) or persistent. A relapse was defined as an evidence of leakage on fluorescein angiography and/or evidence of intraretinal or subretinal fluid on OCT from a previously closed lesion. OCT imaging procedures and fluorescein angiographies were evaluated by three experienced (unmasked) retinal specialists at baseline and during the follow-up, every month for 6 months. Intraocular pressure (IOP), measured by applanation tonometry, was monitored. Diastolic and systolic blood pressures were recorded at baseline and during follow-up. At baseline, the combined therapy group was treated with PDT-V and intravitreal ranibizumab administered on the same day. Monthly, additional ranibizumab injections were administered at month 1 and 2 in case of persistent activity of the neovascular lesion. Group 2 was treated with three monthly injections. Eyes underwent standard PDT-V (Visudyne; Novartis AG, Basel, Switzerland) with the following protocol: a 10-minute infusion of verterporfin (6 mg/m2 body surface area) was followed by its activation (5 minutes later) with a 689-nm diode laser delivering an energy of 50 J/cm2 for 83 seconds, using a spot size of a diameter 1000 ¦Ìm larger than the greatest linear dimension of the lesion. Each patient was asked to wear protective sunglasses and to avoid exposure of the eyes and body surfaces to sunlight for the next 48 hours. They were given an intravitreal injection of 0.5 mg of ranibizumab on the same day. Before the injection, tetracaine 1% eye drops were instilled and povidone-iodine was applied to the eyelid margins and the lashes. After the application of a sterile drape, a lid speculum was inserted and povidone-iodine 5% ophthalmic solution applied to the eye, which was flushed after 90 seconds with saline solution. A volume of 0.5 mg (0.05 ml) of ranibizumab was injected through a 30-gauge needle. Patients self-administered a topical antimicrobial agent (1% ofloxacin ophthalmic solution) three times daily for 3 days before and 7 days after treatment. Follow-up consisted of monthly examinations for a period of 6 months: patients received complete ocular examinations; additionally, BCVA, OCT, IOP, fluorescein angiography, and blood pressure were recorded. Indications for retreatment after the first 3 months were evidence of visual deterioration of at least 5 ETDRS letters together with evidence of persistent or recurrent leakage on fluorescein angiography and/or evidence of intraretinal or subretinal fluid on OCT. Patients with persistence of leakage on OCT and fluorescein angiography but with stable vision, were treated however with additional intravitreal injection of ranibizumab. Group 1 patients requiring further treatment received a second session of PDT combined with intravitreal ranibizumab; Group 2 patients received additional monthly injections. We performed a Wilcoxon rank sum test to compare the BCVA and CMT at T0 and T6 and to determine whether retreatments depended on treatment protocol. The significance level was defined as P < 0.05. | | | Results | A total of 47 eyes of 47 patients were consecutively enrolled and randomly assigned to group 1 (n = 17) or group 2 (n = 30). There were no significant differences in age, pretreatment BCVA, or initial CMT between the groups (Table 1). All the patients completed the 6-month follow-up.
In group 1, the mean baseline BCVA ± standard deviation (SD) was 32.65 ± 11.09 letters (Snellen equivalent, 20/59). Six months after treatment, the mean BCVA was 39.06 ±10.12 letters (20/42). Visual acuity improvement began to appear after the first month of follow-up. The mean BCVA increase was 6.41 ± 13.34 letters. In group 2, the mean baseline BCVA ± SD was 29.13 ± 9.03 letters (20/70). Six months after treatment, the mean BCVA was 33.87 ±12.06 letters (20/57). The mean BCVA increase was 4.73 ± 13.18 letters. In both groups improvement in visual acuity was statistically significant (group 1, P = 0.047; group 2, P = 0.024). There was no statistically significant difference in the BCVA improvement between groups 1 and 2 (Table 2; Figure 1).
In group 1, the mean central thickness at baseline (± SD) was 315 ± 95.49 µm. At 6 months’ follow-up the mean CMT was 202 ± 52.2 µm. In group 2, the mean CMT (± DS) before treatment was of 306 ±71.61 µm. At 6 months’ follow-up the mean CMT was 226 ± 65.58 µm, with a mean CMT reduction of 81 µm (Table 2). In both groups CMT reduction began to appear after the first month of follow-up. In both groups CMT reduction was statistically significant (group 1, P = 0.020; group 2, P = 0.0004). There was no statistically significant difference in the CMT reduction between groups (Table 2; Figure 2).
In group 1, 5 of 17 eyes (29.4%) showed active signs of the membrane (evidence of persistent or recurrent leakage on FA and/or evidence of intraretinal or subretinal fluid on OCT), needing a further cycle of combined therapy, whereas the remaining 13 (71.6%) needed no further treatments. Before treatment, FA shows two areas of leakage with hemorrhagic component and the absence of leakage and resolution of hemorrhagic component 6 months after treatment; at baseline OCT shows intraretinal edema and its resolution 6 month after treatment (Figure 3). The mean number of intravitreal injections was 1.94 (range, 1-4). In group 2, of 30 eyes, 13 (43.3%) needed a further cycle of intravitreal injections. At baseline, FA shows two areas of leakage and the complete resolution of leakage at 6 months of follow-up; before treatment OCT shows an area of hypereflectivity intraretinal corresponding to the intraretinal neovascularitation, associated with serous neurosensory detachment and its complete resolution with return of normal retinal morphology 6 months after treatment (Figure 4).
The mean number of intravitreal injections per eye was 3.4 (range, 3-6). The variation of IOP was not significant (P = 0.57) in both groups (Table 3). There were no serious ocular adverse events such as retinal detachment, endophthalmitis or ocular hypertension. P values of systemic blood pressure were not significant (group 1, 0.33; group 2, 0.17) (Table 4). | |
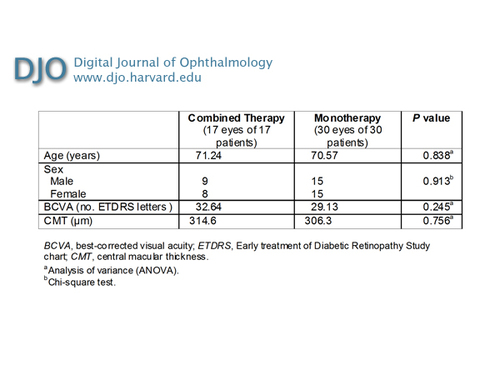
Table 1
Combined therapy versus monotherapy for AMD CNV: patient demographics and comparisons at baseline
|
|
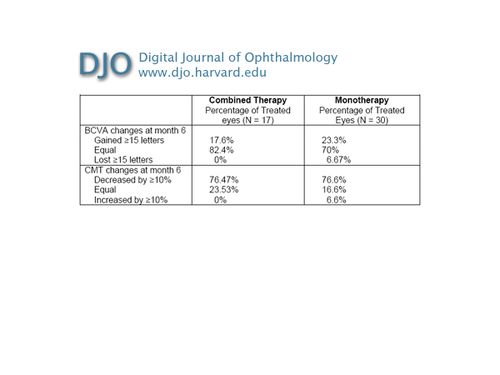
Table 2
Combined therapy versus monotherapy for AMD CNV: visual acuity and optical coherence tomography 6 months after treatment
|
|
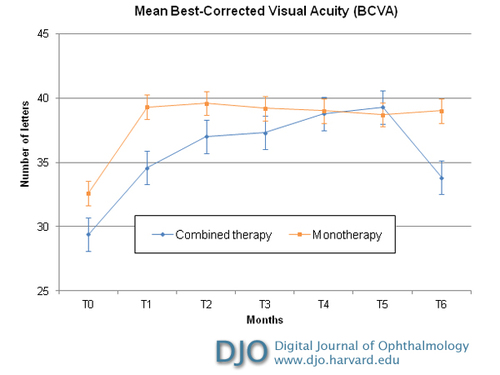
Figure 1
Intravitreal combined therapy versus monotherapy with ranibizumab for choroidal neovascularitation: mean best-correct visual acuity from baseline to month 6 after treatment. The differences in time course between the two subgroups were not significant. Error bars depict the standard deviation around the mean number of letters.
|
|
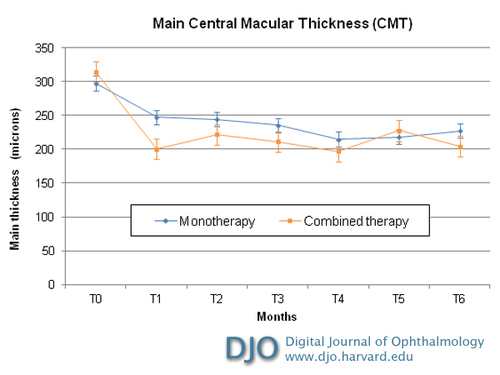
Figure 2
Intravitreal combined therapy versus monotherapy with ranibizumab for choroidal neovascularitation: optical coherence tomography CMT (central macular thickness) from baseline to month 6 after treatment. The differences in time course between the two subgroups were not significant. Errors bars depict the standard deviation around the mean central macular thickness.
|
|
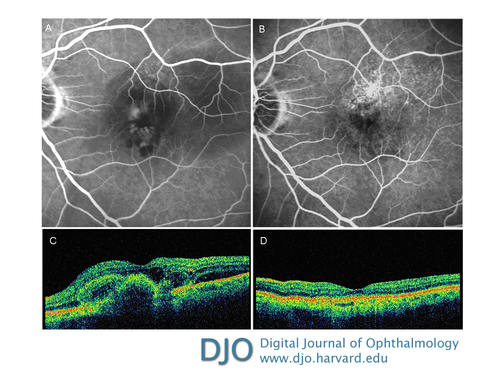
Figure 3
Late-phase fluorescein angiography showing two areas of leakage with hemorrhagic component before treatment with combined therapy (A) and absence of leakage and resolution of hemorrhagic component 6 months after treatment (B). Optical coherence tomography showing an intraretinal area of hyporeflectivity corresponding to the edema associated with areas of hyperreflectivity and indicating neovascularitation prior to combined therapy treatment (C) complete resolution of intraretinal edema with return of normal retinal morphology after treatment (D).
|
|
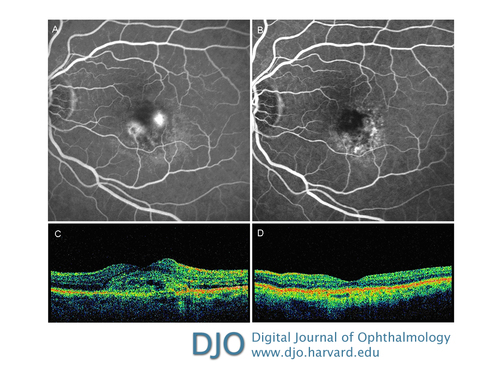
Figure 4
Late-phase fluorescein angiography showing two areas of leakage before monotherapy treatment (A) and 6 months after treatment showing a complete resolution of the leakage (B). Optical coherence tomography before treatment shows an area of hypereflectivity intraretinal corresponding to the intraretinal neovascularitation associated with serous neurosensory detachment (C) and after treatment showing complete resolution of both the neurosensorial detachment and the intraretinal edema with return of normal retinal morphology (D).
|
|
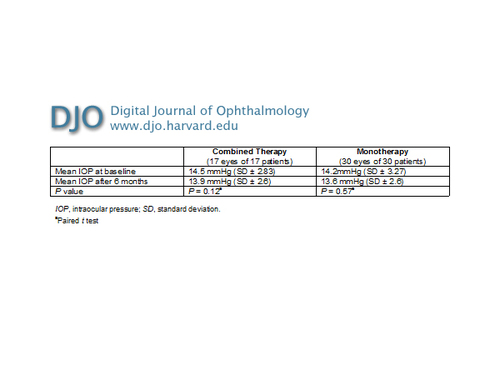
Table 3
Combined therapy versus monotherapy for AMD CNV: IOP at baseline and after 6 months
|
|
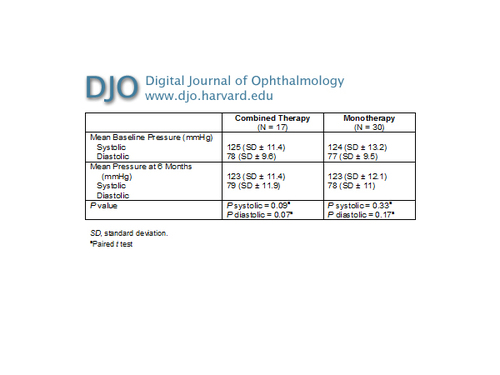
Table 4
Combined therapy versus monotherapy for AMD CNV: blood pressure at baseline and after 6 months
|
|
| Discussion | PDT combines the intravenous injection of a photosensitizer drug with an activating low-power laser beam. PDT has shown good results on classic and predominantly classic subfoveal lesions but has been less effective on occult lesions. PDT-V alone has been shown in a large number of cases to require retreatments every 3 months.(11) Recently, VEGF selective inhibitors have been investigated for treatment of exudative AMD. The "on label" agents used are pegaptanib sodium and ranibizumab.(12) Ranibizumab is a humanized antibody fragment with low molecular weight (48-kDa) that targets all isoforms of VEGF-A, including VEGF-110, VEGF-121, and VEGF-165.(13-14) Although anti-VEGF treatments have been effective, some experimental models have shown that it becomes less effective in preventing the neovascularization and promoting its regression.(15-16) An alternative treatment option is the combined treatment we used for our group 1 patients. This treatment protocol gives PDT-V a new role, in which it may complement anti-VEGF drugs.(16) Combined therapy allows treatment of neovascularization by targeting several key points of the physiopathological processes that are involved in its progression. PDT-V causes the ablation of the already present neovascularization with a phototoxic selective process that destroys endothelial cells and exposes basal membrane.(17) Ranibizumab may block neovascularization relapse with an anti-angiogenetic action toward VEGF-A. A recent study that evaluated VEGF levels in aqueous humor before and one month after combined therapy showed a significant decrease, positively correlated with CMT.(18)
Several multicenter studies are evaluating the effects of the combination of PDT-V with anti-VEGF in patients with subretinal neovascularization. The FOCUS study compared the results of ranibizumab therapy combined with PDT-V and PDT-V alone in patients with subfoveal, predominantly classic CNV, not larger than 5400 µm.(8) In this study, one group of patients had PDT-V and a monthly sham injection and the other PDT-V with a monthly ranibizumab 0.5 mg injection for 24 months. After 24 months, 88% of patients treated with combined therapy and 75% of controls lost fewer than 15 letters. The percentage of patients that has repeated PDT-V was significantly less in the group treated with ranibizumab. The PROTECT study evaluated the efficacy of the combination of a single PDT-V application with a single ranibizumab intravitreal injection administered on the same day and followed by two more injections in 3 months, in eyes with CNV due to AMD. After 9 months of follow-up, 70% (22/33) of eyes had only the scheduled 3 ranibizumab injections and a PDT, whereas 21.8% had an additional PDT treatment with verteporfin.(9) The CAM study showed that the optimal timing for ranibizumab administration is within the 24 hours after PDT.(19)
Kumar et al evaluated the results of combined therapy of a single PDT-V treatment and ranibizumab 0.5 mg received on the same day by 17 patients with CNV due to AMD. Visual acuity was stable (gain/loss <2 lines) in 82.2% of cases, whereas it improved (gain ≥2 lines) in 17.6% of cases.(10) Our combined therapy protocol differs from others in that it provides only one injection at baseline and additional ranibizumab injections at 1 and 2 months to address any persistent activity of the neovascular lesion. In our study, there was no statistically significant difference (P = 0.23) between groups, and both treatments experienced significant improvement in BCVA and CMT. In group 1, the CMT mean reduction was ≥10% in 13 eyes (76.5%). In group 2, the CMT mean reduction was ≥10% in 23 eyes (76.7%). Statistical analysis showed a considerable efficacy of both treatments in reducing CMT (and intraretinal edema) but did not indicate significant advantage in using one or the other (P = 0.06) (Figures 1 and 2) although fewer intravitreal injections were required in group 1 than in group 2. This finding has important ethical and economic implications. Intravitreal injections require a high compliance by the patients, which has to be strictly monitored to avoid serious ocular complications, such as endophthalmitis and retinal detachment. Risk is directly proportional to the number of injections, thus monotherapy carries a greater risk than the combined therapy. Conversely, the risk for patients undergoing the combined therapy is reduced due to the limited number of injections; patients thus have an improved quality of life and psychophysical wellness. In addition, combined therapy reduces the costs of intravitreal injections.(20)
Our study was limited principally by our unmasked protocol as well as the relatively small number of cases and limited follow-up period. One year of follow-up and a larger cohort will provide more conclusive results. | | | Acknowledgements | | This study was supported by the Department of Ophthalmology, University Of Rome "La Sapienza," Rome, Italy. Nicoletta Fantozzi, MD, received a fellowship from Novartis Farma S.p.A.-Origgio, Varese, Italy, from January 1, 2008, to December 31, 2008. No other disclosures are required. | | | References | 1. H. L. Cook, P. J. Patel, and A. Tufai: Age-related macular degeneration: diagnosis and management. British Medical Bulletin 2008;85:127-49.
2. Shah AR, Del Priore LV. Natural history of predominantly classic, minimally classic, and occult subgroups in exudative age-related macular degeneration. Ophthalmology 2009;116:1901-7.
3. Polito A, Isola M, Lanzetta P et al. The natural history of occult choroidal neovascularisation associated with age-related macular degeneration: a systematic review. Ann Acad Med Singapore 2006;35:145-50.
4. Bressler NM; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol 2001;119:198-207.
5. Thys J, Dupont G, Rakic JM. VEGF inhibitors in ophthalmology Rev Med Liege 2009;64: 323-6.
6. Rosenfeld PJ, Brown DM, Heier JS et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration N Engl J Med 2006;355:1419-31.
7. Kaiser PK. Verteporfin photodynamic therapy and anti-angiogenic drugs: potential for combination therapy in exudative age-related macular degeneration. Curr Med Res Opin 2007;23:477-87.
8. Antoszyk AN, Tuomi L, Chung CY, Singh A. FOCUS Study Group. Ranibizumab Combined With Verteporfin Photodynamic Therapy in Neovascular Age-related Macular Degeneration (FOCUS): Year 2 Results Am J Ophthalmol 2008;145:862-74.
9. Schmidt-Erfurth U & Wolf S. PROTECT Study Group. Same-day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularisation due to age-related macular degeneration. Br J Ophthalmol 2008;92:1628-35.
10. Kumar A, Gopalakrishnan K, Sinha S. Combination photodynamic therapy and intravitreal ranibizumab in neovascular AMD in a north Indian population: a pilot study. Retina 2008; 28:1132-7.
11. Wang W, Dean DC, Kaplan HJ.; Changes in visual function and thickness of macula after photodynamic therapy for age-related macular degeneration. Clin Ophthalmol 2010;3:483-8.
12. Diana V Do. Antiangiogenic Approaches to Age-Related. Macular Degeneration in the Future. Ophthalmology 2009;116:S24–S26
13. Martin S Spitzer, Focke Ziemssen, Karl U. Treatment of age-related macular degeneration: focus on ranibizumab. Clinical Ophthalmology 2008;2:1-14.
14. Asensio-Sánchez VM. [Avastin is not the same as Lucentis, and Lucentis is not the same as Avastin] Arch Soc Esp Oftalmol 2009;84:427.
15. Kim IK, Husain D, Michaud N. Effect of intravitreal injection of ranibizumab in combination with verteporfin PDT on normal primate retina and choroid. Invest Ophthalmol Vis Sci 2006;47:357-63.
16. Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology 2008;115:2199-205. Epub 2008 Oct 18.
17. Ju M, Mailhos C, Bradley J. Simultaneous but not prior inhibition of VEGF165 enhances the efficacy of photodynamic therapy in multiple models of ocular neovascularization. Invest Ophthalmol Vis Sci 2008;49:662-70.
18. Patel S.Combination therapy for age-related macular degeneration. Retina 2009;29:S45–S48.
19. Ahn JK, Moon HJ. Changes in aqueous vascular endothelial growth factor and pigment epithelium-derived factor after ranibizumab alone or combined with verteporfin for exudative age-related macular degeneration. Am J Ophthalmol 2009;148:718-724.e1.
20. Debefve E, Pegaz B, Ballini JP. Combination therapy using verteporfin and ranibizumab: optimizing the timing in the CAM model. Photochem Photobiol 2009; 85:1400-8. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in