|
|
 |
 |
 |
 |
|
|
Incidence of postoperative cystoid macular edema by a single surgeon
Digital Journal of Ophthalmology 2009
Volume 15, Number 4
November 28, 2009
|
Printer Friendly
Download PDF |


 Manju L. Subramanian, MD
Manju L. Subramanian, MD | Boston University School of Medicine and Boston VA Hospital Anand K. Devaiah, MD | Boston University School of Medicine Keith A. Warren, MD | University of Kansas School of Medicine
|
|
|
| Abstract | Objective
To evaluate the clinical and angiographic incidence of cystoid macular edema (CME) after cataract surgery, and to determine the impact of intraoperative triamcinolone acetonide.
Methods
This is a prospective, single-center trial looking at 81 eyes of 61 patients who underwent clear-cornea incision phacoemulsification with lens implantation under topical anesthesia by a single surgeon. Outcome measures included clinical and angiographic CME, the impact of operative time, medications, and systemic disease on the presence of CME.
Results
Eight eyes (9.87%) demonstrated angiographic CME at the one-week and six-week follow-up visits. Two eyes showed evidence of clinical CME (2.46%) on examination. Subjects with diabetes had an increased risk of angiographic CME.
Conclusion
The incidence of clinically significant and angiographic CME in this study is confirmatory of previous studies in the literature. The use of intraoperative subconjunctival triamcinolone acetonide did not appear to significantly reduce the development of post-operative CME.
Keywords
Cystoid macular edema, phacoemulsification, cataract surgery, single surgeon, triamcinolone acetonide | | | Introduction | Cystoid macular edema (CME) occurs as a result of vascular leakage attributed to the breakdown of the blood retinal barrier, which is often incited by cataract surgery. CME is characterized by cystoid fluid-filled spaces contained in Henle's layer of the macula. This leakage is easily detected with high sensitivity by fluorescein angiography and optical coherence tomography.
CME is described in 2 ways: angiographic CME and clinically significant CME. Angiographic CME is detected by fluorescein angiogram (FA), and it is often not seen on clinical examination of the eye. Clinically significant CME is observed on biomicroscopic examination and detected on fluorescein angiography. Therefore, angiographic CME is more common than clinical CME.
CME has a peak incidence at four to six weeks following cataract surgery.(1-2) Most cases will spontaneously resolve within weeks to months. The presence of chronic CME occurs in about one percent of patients, leading to chronic or permanent vision loss.
Prospective studies done in the 1960s and 1970s using fluorescein angiography after intracapsular cataract surgery projected the incidence of angiographic CME to be between 36% and 60%.(1-4) More recent prospective studies have shown an incidence of angiographic CME in extracapsular cataract surgery of 10-20%.(5-6) Intraoperative complications leading to acute and prolonged post-operative inflammation, causing breakdown in the blood retinal barrier, have been postulated to have a role in the development of CME. In addition, several studies have established that the presence of systemic vascular disease, such as diabetes mellitus or hypertension, may contribute to further breakdown of the blood retinal barrier and further development of CME.(7-13)
The most current surgical techniques for cataract extraction using phacoemulsification with clear cornea, sutureless incisions and topical anesthesia have led to shorter operative times and reduced phacoemulsification times. Our study prospectively evaluates the clinical and angiographic incidence of post-operative CME in subjects undergoing topical, temporal, clear-cornea phacoemulsification in an ambulatory surgery center by a single surgeon (John D. Hunkeler, MD) in a high-volume private practice with an academic affiliation. We also evaluate the potential impact of intraoperative subconjunctival triamcinolone acetonide on the development of post-operative CME. | | | Materials and Methods | This prospective study was conducted from August 2001 through June 2002. Eighty-one eyes of 61 subjects were enrolled. The study protocol was approved by the Institutional Review Board at the University of Kansas Medical Center.
All patients were determined to have visually significant cataracts by their cataract surgeon. Patients were asked to be part of the study if they met inclusion criteria, which primarily included the presence of a visually significant cataract, informed consent to participate in the study, and compliance with all follow-up visits. Informed consent was obtained by a physician prior to enrollment in the study. Exclusion criteria were as follows: women of childbearing age (unless history of tubal ligation or hysterectomy), allergies to fluorescein dye, pre-existing macular disease, past ocular surgery (ie, refractive surgery, vitreous surgery, trauma), and amblyopia. Patients were also excluded if they had previous or current use of topical pilocarpine or prostaglandin analogs.
All subjects completed a detailed survey outlining their medical and surgical history. They underwent complete dilated eye examinations prior to enrollment. After obtaining informed consent, subjects underwent preoperative fluorescein angiography (FA) to rule-out any pre-existing macular disease. Subjects with diabetes without evidence of retinopathy were included in this study. However, if any macular edema or microangiopathy was seen on fluorescein angiogram preoperatively, they were excluded from the study. Patients with mild drusen in the macula were permitted in the study, but they were excluded if any additional signs of advanced age-related macular changes were seen on clinical exam or fluorescein angiogram.
Subjects then underwent temporal, clear-cornea incision phacoemulsification under topical anesthesia by a single surgeon. The infusion fluid included saline with gentamicin, epinephrine, and vancomycin. A subconjunctival injection of triamcinolone acetonide at a dosage of 16 mg (concentration 40mg/ml) was given in the inferior fornix to each subject at the end of surgery. Post-operatively, patients were placed on a standard regimen of a topical steroid, a topical antibiotic, and a topical non-steroidal anti-inflammatory agent.
Surgical approach, surgical time, phacoemulsification time, and lens type were recorded. Intraoperative complications were carefully documented. All patients were seen at day one, week one, and week six for postoperative examination. A fluorescein angiogram was performed at weeks one and six on every subject to evaluate the presence of angiographic CME, defined as a petalloid pattern of leakage seen in the late phases. Fluorescein angiograms were read by our principal investigator (KAW).
Average visual acuities were calculated using the LogMar equivalent of the best-corrected vision on the Snellen visual acuity chart.(14) Lines of improvement were noted by subtracting the difference between the best-corrected pre-operative vision and the best-corrected final vision at the week 6 post-operative visit. Final visual outcomes and lines improved were compared between the two groups (subjects with CME versus subjects without CME) using student t-tests. Chi-square analysis was used to determine the potential, if any, impact of systemic vascular disease. | | | Results | All 81 patients completed the study requirements and follow-up visits. Ages ranged from 49 to 88 years with the mean age of 71.6. No intraoperative or immediate post-operative complications (such as disruption of the posterior capsule, iris capture, corneal edema, elevated intraocular pressure, choroidal hemorrhage, retinal detachment, etc.), other than CME, were reported. Average pre-operative visual acuity for all subjects (n=81) was 20/42 (20/15 - 20/200). One-week post-operative visual acuity was 20/24 (20/15 - 20/60). Mean best-corrected final vision at 6 weeks was 20/21 (20/15 - 20/70).
Eight eyes (9.87%) demonstrated angiographic CME at either the one-week or six-week follow-up visit. Two eyes (2.5%) showed CME at one week only, 2 (2.5%) eyes showed CME at six weeks only, and 4 (4.9%) eyes at both one and six weeks. Clinically significant CME was noted on biomicroscopic examination in 2 (2.5%) eyes, both at 6 weeks. These two eyes were started on a combination therapy of ketorolac and prednisolone acetate 1%.
The average length of surgery in all eyes (n=81) was 9.22 minutes with a mean phacoemulsification time of 59.85 seconds. The length of surgery in eyes with CME was 10.25 minutes compared with 9.10 minutes in eyes without CME (p=0.24). A mean phacoemulsification time of 52.13 seconds was noted in eyes with CME in contrast to 60.69 seconds in eyes without CME (p=0.31, see Table 1).
All eyes in this study demonstrated a mean of 2.99 lines of improvement on the Snellen visual acuity chart. Eyes with angiographic CME showed a mean of 1.87 lines of improvement, compared to 3.01 lines improvement in eyes without CME (p= 0.18, see Table 1).
Of the 81 eyes included in this study, 6 had a history of diabetes mellitus, 28 had hypertension, 12 had a history of coronary artery disease, and 5 had a history of a prior stroke. Of the eight eyes with angiographic CME, three (37.5%) of the subjects had diabetes mellitus, two (25%) had hypertension, one (12.5%) had a history of coronary artery disease, one (12.5%) had a prior stroke, and one had no significant vascular disease. In subjects without angiographic CME (n=73), three (4.1%) had diabetes mellitus, twenty-six (35%) had hypertension, eleven (15%) had a history of coronary artery disease, and four (5.4%) had a prior stroke (see Table 2).
No major intra-operative complications were noted. Other than the presence of CME, no other post-operative complications were present. Intraocular pressure was not abnormally elevated beyond the immediate post-operative period. | |

Table 1
Mean values for surgical time, phacoemulsification time, pre-operative vision, final vision, and lines of improvement show no statistically significant difference in patients with CME versus those without CME.
|
|
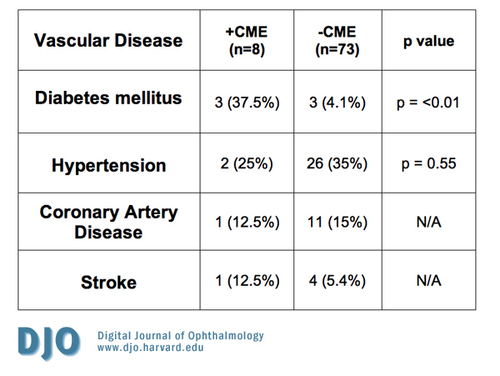
Table 2
Eyes with CME and known vascular disease compared to those without CME and known vascular disease. No statistical difference was noted between the 2 groups for hypertension, coronary artery disease, and stroke. Only diabetes mellitus showed a positive correlation with the presence of CME.
|
|
| Discussion | The incidence of CME after cataract extraction has been studied and reported in the literature. Over time, there has been a decrease in the incidence of this once common post-operative complication. It is often postulated that this decrease in incidence is attributed to improvement in surgical technique, reduced operative times, and less inflammatory intraocular lens devices.
Previous reports have described varying incidence of CME in patients undergoing phacoemulsicifation. In 1996, Lendi et al reported, retrospectively, 4 cases (4.0%) of clinically significant CME.(15) In 1999, Ursell et al reported a 19% incidence of angiographic CME in eyes undergoing cataract extraction via phacoemulsification and concluded the incidence of CME to be similar to that of extracapsular cataract extraction with manual lens removal.(16) In 2003, Mentes et al reported in Ophthalmologica a 9.1% incidence of angiographic CME and no cases of clinical CME after prospectively evaluating 252 eyes undergoing uncomplicated phacoemulsification.(17) More recently, in 2006, Gulkilik et al reported a 25.5% incidence of angiographic CME 10 weeks following temporal, clear cornea phacoemulsification in 98 patients in a prospective study done in Istanbul, Turkey.(18) Of note, a large number of patients with CME in Gulkilik’s study had intraoperative iris trauma during the phacoemulsification, which may have contributed to the seemingly high incidence of development of CME in that study.
The aforementioned studies describe a varying incidence of clinically significant CME and angiographic CME (range 4.0%-25.5%). Some of the studies used different approaches (i.e. scleral tunnel), and all of them involved multiple surgeons. Our study describes a 9.87% incidence of angiographic CME and a 2.5% incidence of clinically significant CME after cataract surgery via temporal, clear-cornea-incision phacoemulsification under topical anesthesia. While our incidence of clinically significant and angiographic CME appears to fall within the range of incidences reported previously in the literature, our prospective study adds additional value to the body of data available in the literature on post-operative CME for 2 reasons. First, the data are obtained in a study by a single surgeon, and second, the application of triamcinolone acetonide at the end of the operation can potentially alter the incidence of the eventual development CME.
In addition, results of our study showed that patients with diabetes are at significantly higher risk for the development of post-operative angiographic CME than those without diabetes, as shown in similar studies.(7-13) Patients with diabetes were enrolled in this study only if the pre-operative fluorescein angiogram showed no evidence of retinopathy. Patients with microangiopathy present on FA were excluded from the study; despite this, our results showed a significantly higher incidence of CME post-operatively in diabetic patients (p=<0.01). Other vascular diseases such as hypertension, coronary artery disease, and a prior history of stroke were not significantly associated with the development of CME.
The impact of intraoperative and peri-operative medications on the development of CME has been variable. It has been suggested that the use of intracameral vancomycin during cataract extraction may increase the risk of post-operative CME.(19-22) Triamcinolone acetonide is often used by vitreoretinal specialists as a potential treatment for post-operative CME, often given through a subtenons or intravitreal approach. However, the impact of subconjunctival triamcinolone acetonide placed in the inferior fornix at the end of the case on the incidence of post-operative, pseudophakic CME is unknown. In our study, no comment can be made on the utility of a single dose of an intraoperative injection of triamcinolone acetonide. Our study did not show an unusually low incidence of post-operative CME as one might intuitively expect with the use of intraoperative triamcinolone acetonide. As such, it can be postulated that the prophylactic use of triamcinolone acetonide in this study may have had no effect on the eventual development of angiographic CME.
This methodology of this study, while prospective in design, has limitations. The lack of a control group presents difficulties when determining if the incidence of CME was reduced by the use of triamcinolone acetonide, as one may expect. It also challenges the role of vancomycin and gentamicin, and its potential impact on the development of post-operative CME. Moreover, while our analysis shows statistical significance and a positive association with respect to CME in patients with diabetes, we acknowledge that the number of patients with diabetes in this study is too small to draw any meaningful conclusions. In addition, the administration of subconjunctival triamcinolone may have delayed the onset of CME, potentially altering the typical time course for post-operative development, as well as possibly missing cases of CME that may have developed and resolved between post-operative visits. Finally, while presenting data on cases from a single surgeon helps to control for any variability in surgical technique, it can be difficult to extrapolate the data and apply it to a teaching institution or a group practice setting.
In summary, this study demonstrated an overall incidence of angiographic CME of 9.87% after topical, clear-cornea phacoemulsification, and a 2.47% incidence of clinically significant CME in all patients who underwent cataract surgery in an outpatient ambulatory surgery center. This study is unique and provides new information for physicians performing cataract surgery in the following ways: First, the data was obtained on operative cases from a single surgeon, and second, the use of subconjunctival triamcinolone acetonide at the end of the operative case did not appear to have any impact on the subsequent development of CME. The single-surgeon data will enable many physicians in the private setting performing high-volume cataract surgery to apply this to their own surgical practice. Further randomized controlled trials evaluating the prophylactic use of depot steroids for prevention of post-operative CME may prove valuable. | | | Acknowledgements | | The authors would like to acknowledge, as a participating investigator, John D. Hunkeler, MD, who was the operating surgeon performing cataract surgery on the study patients. We would also like to acknowledge Clifton C. Davis, MD who obtained informed consent and performed fluorescein angiograms on all of the study patients. | | | References | 1. Jaffe NS, Luscombe SM, Clayman HM, et al. A fluorescein angiographic study of cystoid macular edema. Am J Ophthalmol 1981:92:775.
2. Berrocal JA. Incidence of cystoid macular edema after different cataract operations. Mod Probl Ophthalmol 1977;18:518-20.
3. Meredith TA, Kenyon KR, Singerman LJ, et al. Perifoveal vascular leakage and macular oedema after intracapsular cataract extraction. Br J Ophthalmol 1976; 60(11):765-9.
4. Hagler WS, Manchester TP. Cystoid macular edema after intracapsular cataract extraction and phacoemulsification. J Med Assoc Ga 1977;66(4):225-7.
5. Jaffe NS, Clayman HM, Jaffe MS. Cystoid macular edema after intracapsular and extracapsular cataract extraction with and without an intraocular lens. Ophthalmol 1982;89:25-29.
6. Wright PL, Wilkinson CP, Balyeat HD, et al. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch of Ophthalmol 1988; 106:740-744.
7. Flesner P, Sander B, Henning V, et al. Cataract surgery on diabetic patients. A prospective evaluation of risk factors and complications. Acta Ophthalmol Scand 2002;80(1):19-24.
8. Dowler JG, Hykin PG, Hamilton AM. Phacoemulsification versus extracapsular cataract extraction in patients with diabetes. Ophthalmology 2000;107(3):457-62.
9. Filip M, Stefan C, Cucea R, et al. The vitreoretinal complications of cataract surgery. Oftalmologia 1999;49(4):39-42. Romanian.
10. Bonnet S. Repercussions of cataract surgery on the development of cystoid macular edema in the diabetic patient. Bull Soc Belge Ophtalmol 1995;256:127-9.
11. Menchini U, Bandello F, Brancato R, et al. Cystoid Macular Edema after extracapsular cataract extraction and intraocular lens implantation in diabetic patients without retinoopathy. Br J Ophthalmol 1993:77(4):208-11.
12. Pollack A, Leiba H, Bukelman A, Oliver M. Cystoid macular edema following cataract extraction in patients with diabetes. Br J Ophthalmol 1992;76(4):221- 4.
13. Cheng H, Franklin SL. Treatment of cataract in diabetics with and without retinopathy. Eye 1988;2 (pt 6):6-14.
14. Holladay, JT. Proper Method for Calculating Average Visual Acuity. J of Refract Surg 1997; 13: 388-391.
15. Lendi B., Gonvers M. Phacoemulsification and clear cornea incision: review of 100 initial cases. Klinische-Monatsblatter-fur-Augenheilkunde 1996;208(5):273-4.
16. Ursell, P, Spalton, DJ, Whitcup, SM, Nussenblatt, RB. Cystoid macular edema after phacoemulsification: Relationshop to blood-acqeous barrier damage and visual acuity. J Cat Ref Surg 1999; 25(11):1492-7.
17. Mentes J, Erakgun T, Afrashi F, Kerci G. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica 2003;217(6): 408-12.
18. Gulkilik G, Kocabora S, Taskapili M, Engin G. Cystoid macular edema after phacoemulsification: risk factors and effect on visual acuity. Canadian J of Ophthalmol 2006;41(6):699-703
19. Axer-Siegel R, Stiebel-Kalish H, Rosenblatt I, et al.. Cystoid macular edema after cataract surgery with intraocular vancomycin. Ophthalmol 1999;106(9):1660-4.
20. Gimbel HV, Sun R. Prophylactic intracameral vancomycin and CME. Ophthalmol 2000;107(9):1614-5.
21. Masket S. Prophylactic intracameral vancomycin and CME. Ophthalmol 2000;107(9):1615-6.
22. Blumenthal M. Prophylactic intracameral vancomycin and CME. Ophthalmol 2000;107(9):1616-7. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in