|
|
 |
 |
 |
 |
|
|
Combined Pars Plana Lensectomy/Vitrectomy for Idiopathic Macular Hole Repair Without Postoperative Prone Positioning
Digital Journal of Ophthalmology 2008
Volume 14, Number 3
November 24, 2008
|
Printer Friendly
|


 Nicola G. Ghazi, M.D.
Nicola G. Ghazi, M.D. | University of Virginia Armand Daccache, M.D. | University of Virginia Robert Knape | University of Virginia James S. Tiedeman, M.D., Ph.D. | University of Virginia
|
|
|
| Abstract | Purpose
The aim of this study is to determine the anatomic and visual outcomes following combined pars plana lensectomy/vitrectomy (CPPLV) as a primary procedure for idiopathic macular hole (MH) without post-operative prone positioning (PPP).
Materials and Methods
A retrospective chart review of 42 patients (47 eyes) with MH who underwent CPPLV was performed. No PPP was performed; however, patients were instructed to avoid the supine position during the first postoperative week. The main outcome measures included MH closure rate, best corrected post-operative Snellen visual acuity (BCVA), and procedure complications.
Results
Anatomical closure was achieved in 44 eyes (93.6%). The average BCVA in these eyes improved from 20/203 at baseline to 20/91 post-operatively, with 28 (63.6%) having 20/40 or better. Twenty eight (59.6%) of the 47 eyes had a BCVA of 20/40 or better post-operatively and 35 (74.5%) eyes improved by at least 2 Snellen lines. Post-operative retinal detachment (RD) was observed in 4 eyes (8.5%) and late reopening of the hole in 4 (9.1%).
Discussion
The anatomical and visual outcomes and the RD rate of CPPLV with sulcus intraocular lens implantation without PPP are comparable to those of traditional MH surgery techniques. The main advantages include sparing the patient the inconvenience of PPP and eliminating the need for additional post-vitrectomy cataract extraction procedure.
Keywords
macular hole, pars plana vitrectomy, sulcus intraocular lens (SIOL), post-operative prone positioning | | | Introduction | Idiopathic macular hole (MH) is a retinal condition primarily affecting elderly women with an estimated overall prevalence of 0.14 percent in the Caucasian population.(1,2) Pars plana vitrectomy has been shown to be effective in achieving MH closure and visual improvement.(3) Since the first report of surgical repair of MH by Kelly and Wendel,(4) several modifications of the original technique have been described. The modifications involve many unresolved controversies associated with MH surgery, including the appropriate vitreous substitute used for intraocular tamponade,(5-9) the optimal duration of intraocular tamponade and post-operative prone positioning (PPP) for hole closure,(10-12) the benefits of adjuvant therapy,(13-20) the role of internal limiting membrane (ILM) peeling and the optimal staining material,(21-30) and the extent of vitrectomy needed to achieve MH closure.(31)
Although a duration of up to 4 weeks of intraocular tamponade with a long-acting gas and prone positioning were initially believed to be essential for improved MH closure rate,(10) subsequent reports demonstrated that comparable outcomes were obtained with varying periods of prone positioning, ranging from 1 day to 2 weeks, with or without modification of the surgical technique.(10-13,32-34) Efforts to minimize post-operative positioning arose from the fact that many MH patients with various medical conditions, including arthritis, sleep apnea, and obesity, were poor surgical candidates due to difficulty in complying with postoperative prone positioning. Such patients often require silicone oil (SO) tamponade, though there is increased morbidity associated with SO and subsequent oil removal.(7,9,35,36) Later investigators reported comparable rates of successful MH closure using long-acting gas tamponade without any PPP, thus providing an alternative to the use of SO.(37-39) The incidence of nuclear sclerotic cataract progression in the Vitrectomy for Macular Hole Study was 100 percent at 2 years following vitrectomy and long-acting gas tamponade, despite prone positioning.(40) Therefore, the combination of pars plana vitrectomy (PPV) with long-acting gas tamponade and cataract extraction (CE) into a single procedure for MH repair without PPP, is appealing. The combined surgery eliminates the inconvenience associated with PPP and the use of SO, and avoids subjecting patients to an otherwise inevitable second procedure for post-vitrectomy CE.
In this study, the long-term anatomic and visual results following a new technique combining pars plana lensectomy/vitrectomy (CPPLV) with sulcus intraocular lens implantation (SIOL) as a primary treatment method for idiopathic full thickness macular hole (MH) without PPP is reported. This study describes the advantages of the proposed combination technique over simple pars plana vitrectomy (PPV) and combined phacovitrectomy (CPV). Certain advantages of the CPPLV over PPV are also shared by CPV. | | | Materials and Methods | A retrospective chart review of all patients who underwent CPPLV for macular hole between 1994 and 2006 was performed after obtaining Institutional Review Board (IRB) approval. Table 1 summarizes the inclusion and exclusion criteria for the study. Only patients with the diagnosis of idiopathic macular hole were included. All patients had a complete pre-operative examination, including best-corrected Snellen visual acuity (BCVA), Goldmann applanation intraocular pressure measurement, biomicroscopic anterior segment and retinal evaluation, and binocular indirect ophthalmoscopy. Prior to the availability of optical coherence tomography (OCT), the diagnosis was confirmed by the Watzke-Allen test, stereoscopic fundus photography and/or fluorescein angiography (FA). OCT became the predominant confirmatory test performed after it became available. Data collected included previous ocular history, age, gender, duration of symptoms prior to presentation, post-surgical follow up, BCVA preoperatively and at last follow up, macular hole status postoperatively, and any complications. Data analysis included the conversion of BCVA to the logarithm of the minimal angle of resolution (logMAR) for ease of comparison and analysis. LogMAR visual acuities of all patients at presentation and at last follow-up were obtained, and the means were compared using the t-test for significance.
All study patients had CPPLV performed by one surgeon (JST) using the technique described below. The main outcome measures were the success rate of the procedure, final visual outcome and associated procedure complications. Surgical success was defined as persistent postoperative anatomic closure of the macular hole for at least 2 months following resolution of the intraocular gas tamponade of the macular area.
Surgical procedure
After a three-port pars plana vitrectomy was prepared, the lens was removed first through a pars plana approach using a fragmatome. Following nuclear and cortical removal, the posterior lens capsule was trimmed using the vitreous cutter and an anterior capsulotomy was performed leaving a 360-degree rim of anterior lens capsule with intact zonules. Following the anterior capsulotomy, a core vitrectomy was performed. Separation of the posterior vitreous cortex was induced around the optic nerve and carried out as anteriorly as possible, guided by the “fish-strike” sign. The detached vitreous was removed and the vitreous base trimmed. No attempt was made to peel the ILM.
The sclerotomy sites were then plugged and a 7.5 mm, half-scleral thickness incision was made 2.0 mm posterior to the limbus and tunneled forward. A one-piece, 6.5 mm optic polymethyl methacrylate (PMMA) lens was inserted into the ciliary sulcus and the scleral wound sutured. An air-fluid exchange was performed and the remaining vitreous fluid was allowed 10 minutes to drain posteriorly with the sclerotomy sites plugged. Any re-accumulated fluid was aspirated from the posterior pole in an attempt to achieve a complete air fill. The sclerotomy sites were then sutured closed and a gas exchange was performed using a non-expansile mixture of perfluoropropane (C3F8) gas. No post-operative prone positioning was performed. However, patients were instructed to avoid the supine position during the first post-operative week. Follow-up visits were carried out 1 day, 1 week, 1 month, and 3 months postoperatively and at approximately 6-month intervals afterwards, except in the case of complications when follow-up was performed as needed. | |
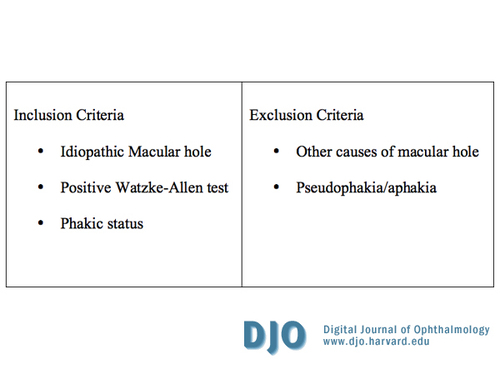
Table 1
Inclusion and exclusion criteria
|
|
| Results | Forty-seven eyes of 42 patients met the inclusion criteria of the study. Patient characteristics are listed in Table 2. Five patients were bilateral cases. The mean age was 67.3 years and 33 patients (70.2%) were women. The duration of symptoms prior to presentation ranged from 1 week to 48 months (mean, 5.6 months). Postoperative follow up varied from 2 to 98 months (mean, 22.7 months). All patients were followed up by the operating surgeon for more than 3 months postoperatively, except for 3 patients who were followed up for only 2 months and subsequently referred back to the referring ophthalmologist for further follow-up because of special circumstances.
Successful MH repair was achieved in 44 (93.6%) of 47 eyes (Figures 1 and 2). Of the 3 eyes that failed the procedure, 2 did not undergo further surgery because of patient preference and 1 was lost to follow-up. One of the 2 patients who declined a second surgery had a parietal lobe meningioma preoperatively with homonymous hemianopsia that contributed to the decision to not pursue further MH repair. Four of the 44 eyes (9.1%) with MH closure had late reopening of the hole at 3, 4, 16 and 18 months, respectively, after successful initial closure. Three of these declined further intervention, while 1 underwent repeat vitrectomy elsewhere with a successful outcome. None of the eyes with late reopening had any detectable evidence of epiretinal membrane (ERM) or macular edema prior to MH reopening.
Table 2 summarizes the postoperative distribution of visual outcome for all 47 study eyes. The proportion of eyes with BCVA of 20/200 or worse decreased from 66.0 percent preoperatively to 19.1 percent postoperatively. Furthermore, 35 (74.5%) of the 47 eyes improved by at least 2 Snellen lines and 28 (59.6%) had a BCVA of 20/40 or better postoperatively. Of the 44 eyes with successful MH closure, the average BCVA improved from about 20/203 at baseline to 20/91 postoperatively (data obtained by converting Snellen acuity to minimal angle of resolution), with 28 (63.6%) of these eyes having 20/40 or better vision. As noted above, 4 of these eyes had late reopening of the MH where the postoperative BCVA changed from 20/20, 20/25, 20/60, and 20/100 to 20/200, 20/200, 20/70 and 20/100 after MH reopening, respectively. In the final analysis, 26 eyes ultimately had a BCVA of 20/40 or better at last follow up visit, corresponding to 55.3 and 59.1 percent of all 47 study eyes and of the 44 eyes with initial successful closure, respectively.
The complications associated with the procedure are summarized in Table 3. There were no cases of endophthalmitis. The most commonly encountered complication was intraocular pressure (IOP) elevation within the first postoperative week, which occurred in 22 eyes (46.8%). The majority of these cases, 14 eyes (63.6%), were controlled with topical eye drops only; 3 eyes required additional systemic carbonic anhydrase inhibitors. In these 17 eyes, the IOP lowering agents were stopped within 3 months postoperatively. In 4 other eyes, IOP elevation was thought to be from gas overfill and the pressure was normalized by “bubble” adjustment on the first postoperative day. The remaining eye had intractable IOP elevation that required tube-shunt implant. Four eyes (8.5%) developed retinal detachment (RD) within 3 months after surgery and those were all repaired with additional surgery. Two of these eyes, one with total RD and another with macula sparing RD but with a childhood history of trauma and unknown visual potential, ended up with a BCVA of 20/200. The remaining 2 eyes had 20/25 final visual outcome. Less frequently observed complications included a visually insignificant epiretinal membrane, extraocular muscle imbalance, cystoid macular edema, temporal visual field defect, capsular phimosis, deposits on the lens optic, localized retinal pigment epithelium irregularity, and superior intraocular lens capture with associated low-grade, anterior segment inflammation, each observed in only 1 eye. The latter resolved completely with a short treatment course of topical prednisolone acetate 1 percent. | |
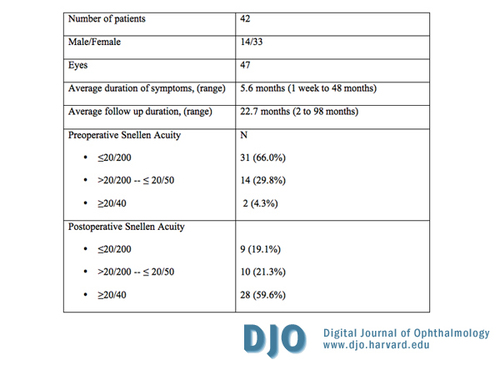
Table 2
Preoperative patient characteristics and visual outcomes following combined pars plana lensectomy/vitrectomy
|
|

Figure 1
Pre-operative optical coherence tomography of an idiopathic macular hole.
|
|

Figure 2
Post-operative optical coherence tomography the idiopathic macular hole seen in Figure 1 after repair by combined pars plana lensectomy/vitrectomy with no post-operative prone positioning. Note anatomical closure of the hole with realignment of retinal layers. Visual acuity improved from 20/200 pre-operatively to 20/30 four months post-operatively.
|
|
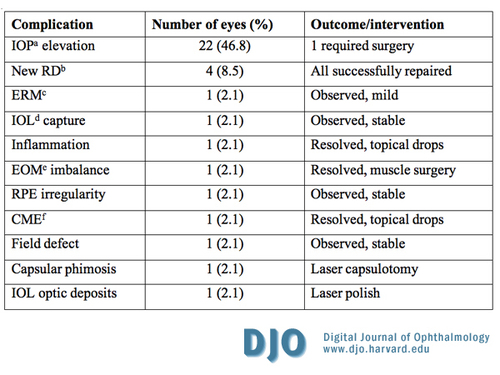
Table 3
Complications following combined pars plana lensectomy/vitrectomy; a, IOP- intraocular pressure; b, RD- retinal detachment; c, ERM- epiretinal membrane; d, IOL- intraocular lens; e, EOM- extraocular muscles; f, CME- cystoid macular edema
|
|
| Discussion | The proposed technique of combining pars plana lensectomy/vitrectomy (CPPLV) with sulcus intraocular lens implantation (SIOL) without PPP for MH repair offers several advantages over traditional surgery for MH repair. The proposed advantages are related to (1) the intrinsic benefits of early lensectomy, (2) the sulcus placement of the IOL, (3) the elimination of PPP and silicone oil (SO) tamponade, and (4) the comparable outcomes of the combination procedure of CPPLV as compared to other methods of MH repair.
Early Lensectomy
Lens removal during MH repair offers several advantages. First, lensectomy eliminates the need for an otherwise inevitable second surgical intervention for a post-vitrectomy cataract extraction (CE) especially given that in the Vitrectomy for Macular Hole Study, the incidence of nuclear sclerosis progression following vitrectomy was 100 percent at 2 years follow-up.(40) Second, early lensectomy avoids the direct surgical challenges associated with delayed CE in vitrectomized eyes.(41-43) Third, avoiding delayed CE following MH repair may help decrease the rate of hole reopening, since delayed CE itself has been implicated as a possible factor in late MH reopening in several studies.(40,44-46) These studies suggest that delayed CE may play a role in late MH reopening; however, the rate of MH reopening of 8.5 percent observed in our study was similar to that noted in the major report by the American Academy of Ophthalmology, which varied between 2 to 10 percent.(47) Fourth, lensectomy virtually avoids the need for laser capsulotomy after the delayed and inevitable CE procedure and its associated potential complications in vitrectomized eyes. In one case-control study comparing phacoemulsification in eyes with past pars plana vitrectomy to eyes without previous vitrectomy, posterior capsule opacification was significantly more common following CE in vitrectomized eyes (51%) than in control eyes (21%).(41) A second study on phacoemulsification and lens implantation after pars plana vitrectomy states, “the most common late complication was the need for neodymium:YAG (Nd:YAG) laser posterior capsulotomy.” (48) Only 2 eyes (4.3%) required YAG laser treatment postoperatively in our study, one due to capsular phimosis and another due to unusual deposits on the intraocular lens optic (Table 3). Fifth, lensectomy allows for early visual rehabilitation and earlier achievement of final visual outcome. Both the initial report of the Vitrectomy for Macular Hole Study and a study by Leonard et al. found that following MH repair, the “improvement in visual function usually is masked in the first postoperative year by progressive nuclear sclerosis.”(3, 49) Also, the major degree of visual improvement primarily occurs soon following CE.(49,50) Sixth, lensectomy, particularly if cataract is present, offers better visualization for intra-and-postoperative management of retinal breaks, RD, ERM dissection, and ILM peeling, if needed. Media opacities in the lens would obscure a direct view of the posterior pole, an issue addressed through both CPPLV and phacovitrectomy, though Kotecha et al. noted decreased posterior visualization in phacovitrectomy.(51) This may be due to the inevitable corneal edema (no matter how mild it is) associated with the anterior delivery of ultrasound energy through an open anterior capsule in phacovitrectomy compared to posterior delivery of ultrasound energy prior to opening the anterior capsule (within the bag) in CPPLV (as performed in our study). The improved visualization may also aid in the early diagnosis and management of posterior segment complications following surgery, which occurred in 41 percent of the patients in the Vitrectomy for Macular Hole Study. Lastly, lens removal during MH repair allows as complete a vitrectomy as possible because of the improved access to the vitreous base. This, in turn, allows as complete an air fill as possible, which is essential when PPP is not performed. Thompson et al. noted that the use of 16 percent C3F8 may allow the gas bubble meniscus to remain larger than 50 percent and may tamponade the MH for at least 1-2 weeks, even in the upright position, when the initial bubble meniscus height was greater than 80 percent of the vitreous cavity.(10) Given those parameters, fluid would only come into contact with the MH during supine positioning within that period. Thus, the greater extent of air fill associated with lens removal makes PPP less critical than in the case of the traditional surgical technique.
Sulcus Placement of the IOL
Implantation of a single-piece intraocular lens in the sulcus (SIOL), as done in this study, instead of a three-piece foldable lens in the capsular bag, as in phacovitrectomy procedures, is also advantageous. We believe that “planned” sulcus implantation, as opposed to “unplanned” sulcus implantation due to unintentional posterior capsular breaks in conventional cataract surgery, of a more rigid single-piece lens offers better stability and support to the IOL, thus limiting forward displacement or excursion of the IOL potentially caused by the gas bubble in the vitreous cavity. The placement of the IOL in the sulcus in turn reduces the possibility of pupillary block glaucoma and allows for a more aggressive intraoperative air fill. Furthermore, lensectomy with SIOL almost eliminates the need for Nd:YAG laser capsulotomy for PCO, which is as common as 75 percent after phacovitrectomy in one study.(52)
The Elimination of PPP and Silicone Oil Tamponade
The CPPLV technique minimizes PPP and allows for avoidance of SO tamponade, an advantage also shared by phacovitrectomy over PPV. In CPPLV, the intra-operative lensectomy allows for better access to the vitreous base and a subsequently improved air fill, making PPP less critical. The optimal duration of PPP remains controversial. Although a duration of up to 4 weeks of intraocular tamponade with a long acting gas and prone positioning was initially believed to be essential for improved MH closure rate (10), similar successful outcomes have been achieved with much shorter periods, ranging from 2 weeks or less,(10-13,32,33,53) to no postoperative positioning at all.(38,39,54) Moreover, PPP may play a role in the development of postoperative IOP elevation (55) and visual field defects,(56) as well as retinal pigment epithelial damage,(37) the most common sight threatening postoperative complication in the Vitrectomy for Macular Hole Study.(16) Furthermore, PPP may be associated with ventilatory disturbances in some patients by decreasing the minute ventilatory volumes and increasing transcutaneous carbon dioxide tensions.(57) More importantly, Foster and Chou reported that the tolerance angle, defined as the cone angle away from the position in which a hole is situated at the apex of a gas bubble, within which a patient can maneuver and still allow for complete hole tamponade, increases with increasing intraocular fill, thus making postoperative positioning less critical.(58) They calculated that for a 60 to 90 percent gas fill, the tolerance angle is about 90 to 120 degrees respectively for holes in the size range of a MH. Therefore, a near complete fill for a MH offers a tolerance angle of greater than 120 degrees, allowing for adequate tamponade without PPP. Their data on the tolerance angle is further supported by the results of this study, which are similar to previously reported data in the literature (Table 4).
A near total gas fill eliminates the need for PPP and allows noncompliant MH patients and those with arthritis, sleep apnea, obesity and other medical conditions, who otherwise require SO tamponade, to be potential candidates for surgery without the need for SO, since PPP compliance is not as critical as with traditional techniques. Avoiding SO eliminates the need for a second procedure for silicone oil removal, and also eliminates the potential ocular morbidity that may be associated with SO. In addition to the known potential complications of SO, sudden unexplained visual loss has been recently reported in several patients with good visual potential following SO removal.(2,35,36) Moreover, SO may accumulate intraretinally, particularly when the ILM has been peeled.(9) Finally, there is evidence indicating that gas tamponade might have a better outcome than SO tamponade in MH repair.(7,8)
Comparable outcomes of CPPLV as Compared to Simple Vitrectomy or Combined Phacovitrectomy (CPV)
The question remains: is performing the combination procedure of CPPLV instead of simple vitrectomy or combined phacovitrectomy (CPV) justifiable, given that the other methods of MH are already proven treatment modalities? The data from our study suggest that CPPLV is an excellent first option. First, in addition to the above mentioned advantages associated with lens removal, the lensectomy is a relatively easy and short portion of the procedure. Second, in a study comparing planned CPPLV to phacovitrectomy for MH repair, the authors noted similar outcomes for both groups of patients, and suggested that CPPLV reduces the operating room time, reduces the cost of the procedure, and offers better visualization of the posterior segment during vitrectomy as compared to phacovitrectomy.(51) Third, only minor complications directly related to the lensectomy portion of the procedure were observed in our series (Table 3), suggesting that the lensectomy itself contributed little morbidity to the overall risk of the combined procedure. No eyes developed chronic anterior uveitis, hyphema, pigment dispersion, posterior synechiae or wound problems. Although the most frequently observed complication of transient postoperative IOP elevation (46.8%) could be partially attributed to anterior segment inflammation, the use of viscoelastic material, and the complete gas fill associated with lensectomy, (an outcome which may also be shared by CPV), there is an even higher rate of postoperative IOP elevation associated with traditional techniques of MH repair without CE (52%).(55) Fourth, and most importantly, the anatomical success rate, visual outcome, and rate of major complications are comparable in our series to those reported in the literature for other techniques of MH repair (Table 4).(16,34,39,47,51,52,54,59,60-62) Gottlieb et al. reported a 78 percent rate of MH closure, with 34 percent of patients having a visual acuity better than 20/40, and a 6 percent rate of retinal detachment, while Lahey et al. reported an 89 percent rate of MH closure, with 65 percent of patients having a visual acuity better than 20/40, and a 3.3 percent rate of retinal detachment.(52,61) By comparison, the current study shows a 93.6 percent rate of MH closure, with 59.6 percent of patients having a visual acuity better than 20/40, and an 8.5 percent rate of retinal detachment. The comparable outcomes, in addition to the potential advantages discussed above, suggest that such a combined technique appears justifiable, though only randomized studies may prove or disprove this notion.
In view of the several advantages and the similar anatomical and visual outcomes and complication rates compared to other techniques of MH repair, CPPLV appears to be a reasonable approach to MH surgery. As noted above, certain advantages of the CPPLV over simple vitrectomy are also shared by CPV, but the overall analysis suggests that CPPLV may also be beneficial over CPV. The described benefits of early lensectomy, a sulcus IOL, the elimination of PPP and SO tamponade, and the comparable outcomes of the combination procedure of CPPLV as compared to other methods of MH repair, all argue for the consideration of CPPLV in the surgical treatment of idiopathic macular hole. | |
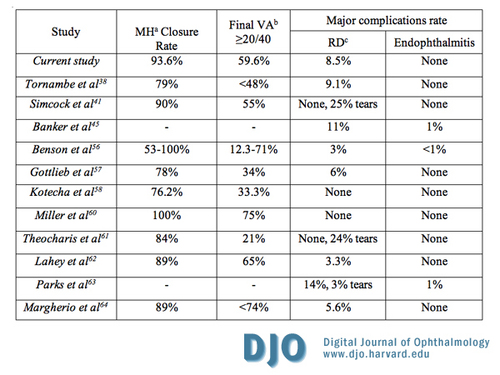
Table 4
Outcomes following combined pars plana lensectomy/vitrectomy compared to the literature; a, MH- idiopathic macular hole; b, VA- visual acuity; c, RD- retinal detachment
|
|
| Acknowledgements | This study was supported by intradepartmental funds through the Lions Sight Foundation of Virginia.
The authors have no proprietary interest.
| | | References | 1. Freeman W. Vitrectomy surgery for full-thickness macular holes. Am J Ophthalmol 1993; 116:233-5.
2. La Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand 2002; 80:579-87.
3. Freeman WR, Azen SP, Kim JW, et al. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol 1997; 115:11-21.
4. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes: Results of a pilot study. Arch Ophthalmol 1991; 109:654-659.
5. Pertile G, Claes C. Silicone oil vs. gas for the treatment of full-thickness macular hole. Bull Soc Belge Ophtalmol 1999; 274:31-6.
6. Goldbaum MH, McCuen BW, Hanneken AM, et al. Silicone oil tamponade to seal macular holes without position restrictions. Ophthalmology 1998; 105:2140-7.
7. Lai JC, Stinnett SS, McCuen BW. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology 2003; 110:1170-4.
8. Couvillion SS, Smiddy WE, Flynn HW Jr, et al. Outcomes of surgery for idiopathic macular hole: a case-control study comparing silicone oil with gas tamponade. Ophthalmic Surg Lasers Imaging 2005; 36:365-71.
9. Chung J, Spaide R. Intraretinal silicone oil vacuoles after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol 2003; 136:766-7.
10. Thompson JT, Smiddy WE, Glaser BM, et al. Intraocular tamponade duration and success of macular hole surgery. Retina 1996; 16:373-82.
11. Krohn J. Duration of face-down positioning after macular hole surgery: a comparison between 1 week and 3 days. Acta Ophthalmol Scand 2005; 83:289-92.
12. Isomae T, Sato Y, Shimada H. Shortening the duration of prone positioning after macular hole surgery- comparison between 1-week and 1-day prone positioning. Jpn J Ophthalmol 2002; 46:84-8.
13. Ezra E, Gregor ZJ. Moorfields Macular Hole Study Group Report No. 1. Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Moorfields Macular Hole Study Group Report No. 1. Arch Ophthalmol 2004; 122:224-36.
14. Trese MT, Williams GA, Hartzer MK. A new approach to stage 3 macular holes. Ophthalmology 2000; 107:1607-11.
15. Saito Y, Tano Y. Intraoperative adjunctive agents in vitrectomy: serum, cytokines, and glue. Semin Ophthalmol 2000; 15:36-43.
16. Banker AS, Freeman WR, Kim JW, et al. Vision-threatening complications of surgery for full-thickness macular holes. Vitrectomy for Macular Hole Study Group. Ophthalmology 1997; 104:1442-52.
17. Paques M, Chastang C, Mathis A, et al. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology 1999; 106:932-8.
18. Olsen TW, Sternberg P Jr, Capone A Jr, et al. Macular hole surgery using thrombin-activated fibrinogen and selective removal of the internal limiting membrane. Retina 1998; 18:322-9.
19. Thompson JT, Smiddy WE, Williams GA, et al. Comparison of recombinant transforming growth factor-beta-2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology 1998; 105:700-6.
20. Korobelnik JF, Hannouche D, Belayachi N, et al. Autologous platelet concentrate as an adjunct in macular hole healing: a pilot study. Ophthalmology 1996; 103:590-4.
21. Kuhn F. Point: to peel or not to peel, that is the question. Ophthalmology 2002; 109:9-11.
22. Hassan TS, Williams GA. Counterpoint: to peel or not to peel: is that the question? Ophthalmology 2002; 109:11-2.
23. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol 2000; 129:769-77.
24. Kimura T, Takahashi M, Takagi H, et al. Is removal of internal limiting membrane always necessary during stage 3 idiopathic macular hole surgery? Retina 2005; 25:54-8.
25. Alpatov S. Is removal of internal limiting membrane always necessary during stage 3 idiopathic macular hole surgery? Retina 2005; 25:949, author reply.
26. Cheung CM, Munshi V, Mughal S, et al. Anatomical success rate of macular hole surgery with autologous platelet without internal-limiting membrane peeling. Eye 2005; 19:1191-3.
27. Kadonosono K, Itoh N, Uchio E, et al. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol 2000; 118:1116-8.
28. Lee KL, Dean S, Guest S. A comparison of outcomes after indocyanine green and trypan blue assisted internal limiting membrane peeling during macular hole surgery. Br J Ophthalmol 2005; 89:420-4.
29. Gale JS, Proulx AA, Gonder JR, et al. Comparison of the in vitro toxicity of indocyanine green to that of trypan blue in human retinal pigment epithelium cell cultures. Am J Ophthalmol 2004; 138:64-9.
30. Karacorlu M, Ozdemir H, Arf Karacorlu S. Does intravitreal triamcinolone acetonide-assisted peeling of the internal limiting membrane effect the outcome of macular hole surgery? Graefes Arch Clin Exp Ophthalmol 2005; 243:754-7.
31. Spaide RF. Macular hole repair with minimal vitrectomy. Retina 2002; 22:183-6.
32. Ellis JD, Malik TY, Taubert MA, et al. Surgery for full-thickness macular holes with short-duration prone posturing: results of a pilot study. Eye 2000; 14:307-12.
33. Mulhern MG, Cullinane A, Cleary PE. Visual and anatomical success with short-term macular tamponade and autologous platelet concentrate. Graefes Arch Clin Exp Ophthalmol 2000; 238:577-83.
34. Margherio RR, Margherio AR, Williams GA, et al. Effect of perifoveal tissue dissection in the management of acute idiopathic full thickness macular holes. Arch Ophthalmol 2000; 118: 495-98.
35. Newsom RS, Johnston R, Sullivan PM, et al. Sudden visual loss after removal of silicone oil. Retina 2004; 24:871-7.
36. Cazabon S, Groenewald C, Pearce IA, Wong D. Visual loss following removal of intraocular silicone oil. Br J Ophthalmol 2005; 89:799-802.
37. Poliner LS, Tornambe PE. Retinal pigment epitheliopathy after macular hole surgery. Ophthalmology 1992; 99:1671-7.
38. Szurman P, Di Tizio FM, Lafaut B, et al. Significance of postoperative face-down positioning after surgery for idiopathic macular holes: consecutive case-control study. Klin Monatsbl Augenheilkd 2000; 217:351-5.
39. Simcock PR, Scalia S. Phacovitrectomy without prone posture for full thickness macular holes. Br J Ophthalmol 2001; 85:1316-9.
40. Cheng L, Azen SP, El-Bradey MH, et al. Duration of vitrectomy and postoperative cataract in the vitrectomy for macular hole study. Am J Ophthalmol 2001; 132:881-7.
41. Pinter SM, Sugar A. Phacoemulsification in eyes with past pars plana vitrectomy: case-control study. J Cataract Refract Surg 1999; 25:556-61.
42. Biro Z, Kovacs B. Results of cataract surgery in previously vitrectomized eyes. J Cataract Refract Surg 2002; 28:1003-6.
43. Chang MA, Parides MK, Chang S, Braunstein RE. Outcome of phacoemulsification after pars plana vitrectomy. Ophthalmology 2002; 109:948-54.
44. Paques M, Massin P, Blain P, et al. Long-term incidence of reopening of macular holes. Ophthalmology 2000; 107:760-5.
45. Paques M, Massin P, Santiago PY, et al. Late reopening of successfully treated macular holes. Br J Ophthalmol 1997; 81:658-62.
46. Christmas NJ, Smiddy WE, Flynn HW Jr. Reopening of macular holes after initially successful repair. Ophthalmology 1998; 105:1835-8.
47. Benson WE, Cruickshanks KC, Fong DS, et al. Surgical management of macular holes: a report by the American Academy of Ophthalmology. Ophthalmology 2001; 108:1328-35.
48. Grusha YO, Masket S, Miller KM. Phacoemulsification and lens implantation after pars plana vitrectomy. Ophthalmology 1998; 105:287-94.
49. Leonard RE 2nd, Smiddy WE, Flynn HW Jr, Feuer W. Long-term visual outcomes in patients with successful macular hole surgery. Ophthalmology 1997; 104:1648-52.
50. Scott IU, Moraczewski AL, Smiddy WE, et al. Long-term anatomic and visual acuity outcomes after initial anatomic success with macular hole surgery. Am J Ophthalmol 2003; 135:633-40.
51. Kotecha AV, Sinclair SH, Gupta AK, Tipperman R. Pars plana vitrectomy for macular holes combined with cataract extraction and lens implantation. Ophthalmic Surg Lasers 2000; 31:387-93.
52. Gottlieb CC, Martin JA. Phacovitrectomy with internal limiting membrane peeling for idiopathic macular hole. Can J Ophthalmol 2002; 37:277-82.
53. Park DW, Sipperley JO, Sneed SR, et al. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology 1999; 106:1392-7.
54. Tornambe PE, Poliner L, Grote K. Macular hole surgery without face-down positioning. Retina 1997; 17:179–85.
55. Chen CJ. Glaucoma after macular hole surgery. Ophthalmology 1998; 105:94-100.
56. Boldt HC, Munden PM, Folk JC, Mehaffey MG. Visual field defects after macular hole surgery. Am J Ophthalmol 1996; 122:371-381.
57. Matsuda M, Yoneda J, Nakamura M, et al. Prevention of ventilatory disturbance while in the face-down position after macular hole surgery. Nurs Health Sci 2002; 4:85-8.
58. Foster WJ, Chou T. Physical mechanisms of gas and perfluoron retinopexy and subretinal fluid displacement. Phys Med Biol 2004; 49:2989-97.
59. Miller JH Jr, Googe JM Jr, Hoskins JC. Combined macular hole and cataract surgery. Am J Ophthalmol 1997; 123:705-7.
60. Theocharis IP, Alexandridou A, Gili NJ, Tomic Z. Combined phacoemulsification and pars plana vitrectomy for macular hole treatment. Acta Ophthalmol Scand 2005; 83:172-5.
61. Lahey JM, Francis RR, Fong DS, et al. Combining phacoemulsification with vitrectomy for treatment of macular holes. Br J Ophthalmol 2002; 86:876-8.
62. Park SS, Marcus DM, Duker JS, et al. Posterior segment complication after vitrectomy for macular hole. Ophthalmology 1995; 102:775–81. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in