|
|
 |
 |
 |
 |
|
|
Surgical outcome and pathological findings in macular epiretinal membrane caused by neurofibromatosis type 2
Digital Journal of Ophthalmology
2022
Volume 28, Number 1
January 31, 2022
|
Printer Friendly
Download PDF |
|
|


 Hiroshi Kunikata, MD, PhD
Hiroshi Kunikata, MD, PhD | Department of Ophthalmology, Tohoku University Graduate School of Medicine; Department of Retinal Disease Control, Tohoku University Graduate School of Medicine Koji M. Nishiguchi, MD, PhD | Department of Advanced Ophthalmic Medicine, Tohoku University Graduate School of Medicine Mika Watanabe, MD, PhD | Department of Pathology, Tohoku University Graduate School of Medicine Toru Nakazawa, MD, PhD | Department of Ophthalmology, Tohoku University Graduate School of Medicine; Department of Retinal Disease Control, Tohoku University Graduate School of Medicine; Department of Advanced Ophthalmic Medicine, Tohoku University Graduate School of Medicine; Department of Ophthalmic Imaging and Information Analytics, Tohoku University Graduate School of Medicine
|
|
|
| Abstract | | We present surgical outcomes in a 10-year-old Japanese girl with neurofibromatosis type 2 (NF2)–induced epiretinal membrane (ERM). Her right eye underwent lens-sparing 27-gauge microincision vitrectomy surgery (MIVS) with ERM peeling. Decimal best-corrected visual acuity increased from 0.3 to 0.4 postoperatively. However, abnormal thickening of the macula persisted for 3 years. Staining of the extracted ERM revealed many cells positive for glial fibrillary acidic protein and nestin. Although removal of NF2-induced ERM with MIVS can improve visual acuity, the potential surgical risks require careful consideration on a case-by-case basis. | | | Introduction | Neurofibromatosis type 2 (NF2), characterized by multiple inherited schwannomas, meningiomas, and ependymomas, arises from a mutation on chromosome 22.(1) It can cause benign intracranial tumors in the nerve sheath of the auditory-vestibular nerve or spinal cord, causing hearing loss or weakness in the limbs.(1-4) In the eye, early-onset NF2 often causes cataracts and ERM.(5) The prevalence of ERMs in NF2 patients is approximately 80%,(2,4) increasing with the severity of the mutation.(6) Unique features of ERMs may permit early diagnosis in neurologically asymptomatic children with a severe NF2 phenotype.(5,7-9)
One possible treatment for NF2-related ERMs is peeling with vitreoretinal surgery. However, since NF2 is very rare (1/100,000),(1) and vitreoretinal surgery is generally difficult in children, the postoperative visual prognosis and the pathological condition of children with NF2-related ERMs remains unclear. We present a case, with histological analysis, of ERM removal in a pediatric patient with NF2.
| | | Case Report | A 10-year-old Japanese girl with NF2 presented at Tohoku University Hospital for evaluation and treatment. Bilateral visual disturbance and ERMs were present since she was 3 years of age. Despite eye patch therapy, decimal best-corrected visual acuity was 0.3 in the right eye. A diagnosis of NF2 was made at age 9 years, when the patient underwent surgery for a cervical spinal cord tumor.
Neither eye had high myopia or an abnormal anterior segment. The right eye had a thickened ERM, with raised edges extending into the vitreous cavity, occupying a macular area of 1.5 disc diameters (Figure 1). The ERM caused a loss of foveal contour, leading to disorganization of the inner and outer retinal layers (Figure 1). The left eye had a mild, fovea-sparing ERM.
The patient underwent lens-sparing 27-gauge microincision vitrectomy surgery (27 G MIVS) in the right eye under general anesthesia at 10 years of age (Figure 2). The vitreous was firmly attached at the posterior pole and peripheral retina; therefore, we created a posterior vitreous detachment (PVD) and applied triamcinolone acetonide (MaQuaid, Wakamoto Pharmaceutical, Tokyo, Japan). There were multiple peripheral tears, with slight bleeding, probably due to the PVD. Endolaser photocoagulation was used to seal the tears. The ERM was successfully peeled and partially cut with scissors where it was integrated with the macula. Peeling of the internal limiting membrane (ILM) was not attempted during surgery.
Histological analysis of the resected ERM revealed many cells of indeterminate origin. Staining of the sample was negative for S100 protein, weakly positive for glial fibrillary acidic protein (GFAP) and moderately positive for nestin (Figure 3). The specimen was too small to stain for periodic acid-Schiff, type IV collagen, laminin, CD44, and vascular endothelial growth factor.
Postoperatively, decimal best-corrected visual acuity improved from 0.3 to 0.4 in the right eye. Central macular thickness did not notably change, and the abnormal macular thickening persisted for 3 years after surgery (Figure 1). Postoperatively, retinal sensitivity (RS) increased in most areas of the macula but decreased in the upper temporal parafovea (Figure 1). There were no complications and no ERM recurrence 3 years after surgery. | |
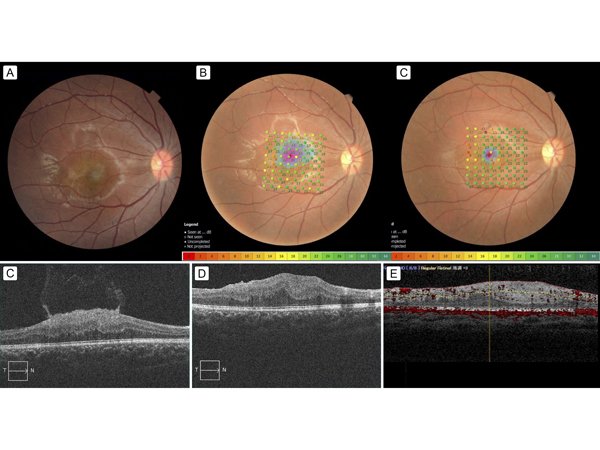
Figure 1.
Pre- and postoperative fundus photographs, with MP-3 stimulation results and optical coherence tomography (OCT) findings. A, Preoperative color fundus photograph of the right eye showing a gray, thickened epiretinal membrane (ERM), occupying a macular area of 1.5 disc diameters. B, Preoperative color fundus photograph showing the 64 microperimetry test points in the macular region as green or yellow points; many macular points had mildly decreased retinal sensitivity (RS), as shown in yellow (RS measured with microperimetry [MP-3; Nidek, Japan]). C, Three-year postoperative color fundus photograph; most of the yellow points in the macula shown in B had changed to green, ie, RS had increased, but some points in the upper temporal macula changed to orange or red, ie, had decreased RS postoperatively. D, Preoperative OCT scans showing a thickened ERM with a hyper-reflective surface; this is a characteristic appearance of neurofibromatosis type 2 (NF2)–related ERMs: flame-shaped or with a spiculated appearance and curled edges that project anteriorly into the vitreous. OCT-measured central macular thickness (CMT) was 531 μm (Cirrus HD-OCT; Carl Zeiss Meditec, Oberkochen, Germany). E, One-month postoperative OCT scan showing the absence of the ERM and the reduction of the hyper-reflective surface; however, the central macula was still abnormally thickened. OCT-measured CMT was 384 μm (Cirrus HD-OCT). F, Three-year postoperative OCT scan showing the absence of the ERM; however, the central macula was still abnormally thickened, and OCT-measured CMT was 563 μm (RS-3000 advance; Nidek, Japan).
|
|
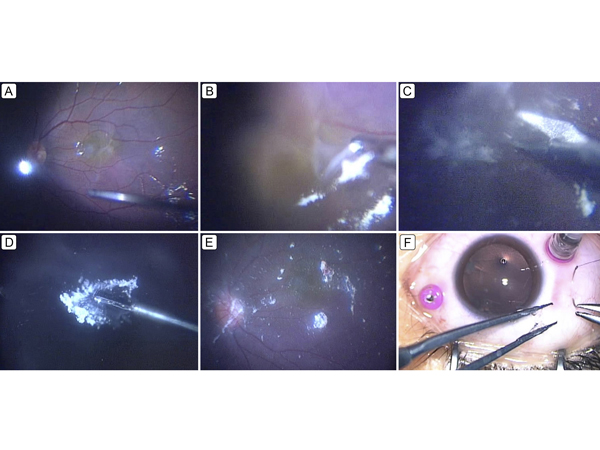
Figure 2.
Intraoperative surgeon’s view of 27-gauge microincision vitrectomy surgery (27 G MIVS) for NF2-related ERM peeling. A, The vitreal core was resected, and preservative-free triamcinolone acetonide (MaQaid; Wakamoto Pharmaceutical Co Ltd, Tokyo, Japan) was injected into the vitreous cavity, after which a posterior vitreous detachment was created. The presence of an ERM was clearly confirmed. B, Most of the ERM was easily peeled, but part of it was strongly adherent to the temporal region of the macula and seemed to have become integrated with it. C, To avoid damaging the macula, the ERM was cut at the temporal region with scissors. D, The ERM was successfully removed from the macula. E, After removal of the ERM, it was confirmed that no remnant ERM was in the macula. F, The scleral ports created during 27 G MIVS were closed with absorbable sutures at the end of the surgery.
|
|
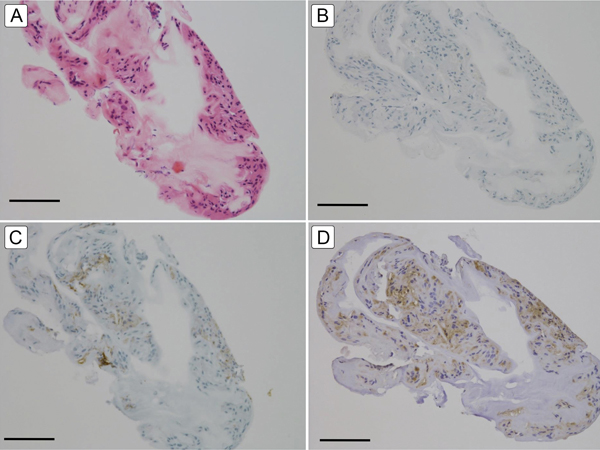
Figure 3.
Light microscope images of surgically removed NF2-related ERM tissue. A, Hematoxylin-eosin staining showing the presence of many cells in the ERM tissue (bar = 100 μm). B, Negative staining for S100 protein (bar = 100 μm). C, Weakly positive staining for glial fibrillary acidic protein (bar = 100 μm). D, Moderately positive staining for nestin (bar = 100 μm).
|
|
| Discussion | To our knowledge, postoperative visual outcomes in patients with NF2-related ERMs have been reported for only 2 cases (Table 1).(10,11) Han et al described an ERM in a 2-year-old girl that adhered strongly to the macula but had a clearly defined border with the retina.(10) Unfortunately, postoperative visual improvement was not reported. Philip et al reported a 6-year-old boy who underwent 23 G MIVS with ERM peeling.(11) Five months postoperatively, his best-corrected visual acuity had improved, and his performance at school improved remarkably.(11)
There are no standard guidelines for treating NF2 patients with ERMs, but observation and surgical intervention are common treatment choices.(9) Young children with ERMs or retinal hamartomas that are not specifically related to NF2 have shown good visual recovery after early vitrectomy.(12,13) The current report shows that an NF2-related ERM affected visual acuity, and that surgery slightly but stably increased visual acuity for at least 3 years. Furthermore, our patient showed increased RS at most points in the macula, despite decreased postoperative RS in some parafoveal points. It may be that peeling the ERM, which in this patient was tightly integrated and had to be removed with the aid of scissors, caused retinal damage. We believe that the ERM could only have been completely removed with scissors, regardless of which MIVS gauge had been used, because the ERM was integrated so deeply into the retina. Additionally, the vitreous was strongly attached to the retina, and peripheral retinal tears formed during PVD creation, as in a previously reported case.(11) Maximum caution is necessary during vitreoretinal surgery for ERM peeling in NF2 patients, to avoid retinal damage or tears. Lastly, it is important to note that the abnormally thickened macula did not markedly change postoperatively, even after 3 years. The two previous surgical reports described similar persistent structural abnormalities.(10,11)
Though ERMs related to NF2 are generally considered to be combined hamartomas of the retina and the retinal pigment epithelium, they have not been precisely defined because of their rarity. To our knowledge, only 4 published reports have included histological findings (Table 1).(2,10,11,14) Two of these reports obtained histopathological results via autopsy, not vitrectomy, and were published more than 10 years ago.(2,14) In 1992, Kaye et al described an NF2 patient with intraretinal glial proliferation and an overlying ERM that was composed of astrocytic cells and stained positively for GFAP.(2) In 1995, Crawford reported that numerous defects were present in the ILM of an eye with NF2, and that glial proliferation formed plaques on the ILM.(4) In 2007, McLaughlin et al described an NF2 patient with a thin ERM comprised of spindled and cuboidal cells that were focally positive for S100 protein and GFAP, suggesting a glial origin.(14) In the current report, the extracted ERM stained positively for GFAP and nestin, which is characteristic of Müller cells, a type of retinal glial cell. Nestin has been reported to upregulate in primary retinal Müller cells in response to inflammation and angiogenesis after retinal injuries.(15) Thus, we speculate that the origin of the ERM in patients with NF2 is the retinal Müller cells.
In conclusion, although surgical removal of an ERM, possibly originating from retinal glial cells, in a pediatric patient with NF2 improved long-term visual acuity, the outcome was somewhat limited by persistent morphological abnormality of the macula. Additionally, we recommend caution when peeling NF2-related ERMs in children, to minimize intraoperative peripheral retinal tearing and a postoperative decrease in parafoveal RS. Pediatric eyes differ from adult eyes, and creating a PVD always requires extra care and attention. Considering the potential risks, vitreous surgery for NF2-related ERMs in children should be performed only after careful assessment of the risk and should be used on a case-by-case basis.
Literature Search
PubMed was searched on January 1, 2021, for English-language results, using the following terms and combinations: surgical outcome, epiretinal membrane, and neurofibromatosis type 2.
Acknowledgments
This paper was supported in part by a JST grant from JSPS KAKENHI Grants-in-Aid for Scientific Research (C) (HK 40361092). The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data. | |
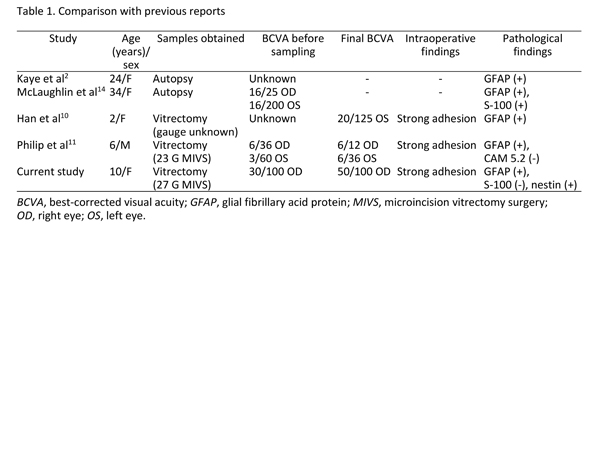
Table 1.
Comparison with previous reports
|
|
| References | 1. Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet 2009;373:1974-86.
2. Kaye LD, Rothner AD, Beauchamp GR, Meyers SM, Estes ML. Ocular findings associated with neurofibromatosis type II. Ophthalmology 1992;99:1424-9.
3. Landau K, Yasargil GM. Ocular fundus in neurofibromatosis type 2. Br J Ophthalmol 1993;77:646-9.
4. Meyers SM, Gutman FA, Kaye LD, Rothner AD. Retinal changes associated with neurofibromatosis 2. Trans Am Ophthalmol Soc 1995;93:245-57.
5. Bosch MM, Boltshauser E, Harpes P, Landau K. Ophthalmologic findings and long-term course in patients with neurofibromatosis type 2. Am J Ophthalmol 2006;141:1068-77.
6. Painter SL, Sipkova Z, Emmanouil B, Halliday D, Parry A, Elston JS. Neurofibromatosis type 2-related eye disease correlated with genetic severity type. J Neuroophthalmol 2019;39:44-9.
7. Sisk RA, Berrocal AM, Schefler AC, Dubovy SR, Bauer MS. Epiretinal membranes indicate a severe phenotype of neurofibromatosis type 2. Retina 2010;30:S51-8.
8. Waisberg V, Rodrigues LO, Nehemy MB, Frasson M, de Miranda DM. Spectral-domain optical coherence tomography findings in neurofibromatosis type 2. Invest Ophthalmol Vis Sci 2016;57:OCT262-7.
9. Giovinazzo JV, Rosen R, Gupta M. A young girl with bilateral atypical epiretinal membranes. JAMA Ophthalmol 2019;137:571-2.
10. Han DP, Chin M, Simons KB, Albert DM. Surgical removal of an atypical macular epiretinal membrane in neurofibromatosis type 2: clinicopathologic correlation and visual outcome. Arch Ophthalmol 2012;130:1337-9.
11. Philip SS, Kuriakose T, Chacko G. Successful surgical management of bilateral epiretinal membrane in a child with only cafe-au-lait spots. Indian J Ophthalmol 2017;65:531-3.
12. Ferrone PJ, Chaudhary KM. Macular epiretinal membrane peeling treatment outcomes in young children. Retina 2012;32:530-6.
13. Bonnin S, Metge F, Guez A, Edelson C, Dureau P, Caputo G. Long-term outcome of epiretinal membrane surgery in young children. Retina 2016;36:558-64.
14. McLaughlin ME, Pepin SM, Maccollin M, Choopong P, Lessell S. Ocular pathologic findings of neurofibromatosis type 2. Arch Ophthalmol 2007;125:389-94.
15. Hosoki A, Oku H, Horie T, et al. Changes in expression of nestin, CD44, vascular endothelial growth factor, and glutamine synthetase by mature muller cells after dedifferentiation. J Ocul Pharmacol Ther 2015;31:476-81. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in