|
|
 |
 |
 |
 |
|
|
Rapid growth of primary uveal melanoma following intravitreal bevacizumab injection: a case report and review of the literature
Digital Journal of Ophthalmology
2020
Volume 26, Number 3
July 3, 2020
DOI: 10.5693/djo.02.2020.06.001
|
Printer Friendly
Download PDF |
|
|



Jingyi Ma, BMSc | Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada Kelsey A. Roelofs, MD, FRCSC | Department of Ophthalmology and Visual Sciences, University of Alberta, Edmonton, Alberta, Canada Laurie Russell, MD, FRCPC | Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, Alberta, Canada Ezekiel Weis, MD, MPH, FRCSC | Department of Ophthalmology and Visual Sciences, University of Alberta, Edmonton, Alberta, Canada; Department of Surgery, University of Calgary, Calgary, Alberta, Canada Sylvia H Chen, MDCM, MBA, FRCSC | Department of Ophthalmology and Visual Sciences, University of Alberta, Edmonton, Alberta, Canada
|
|
|
| Abstract | | Uveal melanoma size is a significant predictor of tumor metastasis. Although the relationship between antivascular endothelial growth factors (VEGF) and uveal melanoma growth has been studied, results are paradoxical, and the relationship remains controversial. We report the case of a 65-year-old man who presented with elevated intraocular pressure in his right eye, neovascularization of his iris, and significant corneal edema, which obscured the view of the angle. Given his history of proliferative diabetic retinopathy, he was diagnosed with neovascular glaucoma and subsequently received an intravitreal injection of bevacizumab and underwent Ahmed valve insertion. This was complicated by postoperative hyphema. Two and a half months postoperatively, a mass involving the inferior iris and ciliary body became visible, and fine-needle aspiration biopsy confirmed uveal melanoma. Seven weeks after diagnosis, the tumor’s largest basal diameter had increased from 2.51 mm to 18.0 mm, and apical height increased from 6.23 mm to 11.0 mm. His right eye was enucleated. Histopathological analysis showed discontinuous invasion next to the Ahmed valve. Tumor progression after injection raises the possibility that in some untreated uveal melanomas, accelerated growth may occur following exposure to anti-VEGF agents. | | | Introduction | | Uveal melanoma most commonly originates from the choroid; however, tumors arising in the ciliary body tend to behave more aggressively and carry a worse prognosis.(1,2) Larger tumor size is a significant predictor of metastasis,(1,2) independent of other characteristics, including gene expression profile.(3) Additionally, higher mitotic index, mixed cell type, and epithelioid cell type melanomas may be associated with faster rates of growth.(4,5) We present the case of a patient who demonstrated uncharacteristically rapid ciliary body melanoma growth following an injection of intravitreal bevacizumab for neovascular glaucoma (NVG), presumed secondary to proliferative diabetic retinopathy. We also summarize the literature on the use of drugs targeting vascular endothelial growth factors (VEGF) to treat uveal melanoma. | | | Case Report | A 65-year-old man with a history of proliferative diabetic retinopathy (PDR) status post panretinal photocoagulation (PRP) presented to the Retina Service at the Royal Alexandra Hospital with complaints of right eye irritation and blurred vision. On routine PDR examination 1.5 months prior, his eye examination was noted to be unremarkable, with four quadrants of PRP noted in both eyes. On this repeat examination, his right eye was noted to have 2+ microcystic cornea edema, temporal neovascularization of his iris, and a mild vitreal hemorrhage in the presence of prior PRP. Intraocular pressure (IOP) was elevated to 36 mm Hg, and angle assessment was limited by a poor view through his cornea. He was diagnosed with NVG secondary to PDR and received an intravitreal injection of bevacizumab (1.25 mg in 0.05 mL). Because his IOP remained markedly elevated after injection despite maximal topical glaucoma therapy and acetazolamide, he was referred to the Glaucoma Service 3 days later and underwent semiurgent Ahmed tube shunt insertion 7 days later. The surgery and initial postoperative course were unremarkable, aside from a persistent anterior chamber hyphema. At postoperative follow-up 2.5 months later, the hyphema had cleared, and an inferior mass involving the iris and ciliary body was visible (Figure 1A).
The patient was referred to the Ocular Oncology Service, and a fine-needle aspiration biopsy was performed to determine whether the mass was melanoma or metastatic carcinoma. Histopathology revealed malignant epithelioid-like cells, some with cytoplasmic pigment (Figure 2A), consistent with uveal melanoma, although metastatic carcinoma could not be excluded. Because the clinical presentation was atypical, the patient underwent a computed tomography scan of his chest and abdomen 10 days later to rule out a primary carcinoma elsewhere. The scan identified multiple lung and liver lesions. The patient underwent a liver biopsy and was referred to Medical Oncology for consideration of palliative chemotherapy. Four weeks later, the liver biopsy showed melanoma, confirming the eye as the primary site, after which the patient underwent enucleation. The underlying etiology for NVG was revised from presumed proliferative diabetic retinopathy to tumor-induced ischemia. At enucleation, the tumor’s largest basal diameter had increased dramatically, from 2.51 mm to 18.0 mm, as did its apical height, from 6.23 mm to 11.0 mm. Prior to enucleation, anterior segment photographs were obtained to document this growth (Figure 1B). On staging investigations, liver metastases were present. Histopathological analysis confirmed malignant melanoma stage pT3d pN1b pM1a, with discontinuous invasion next to the Ahmed valve (Figure 2B-C). Given the lung and liver metastases at the time of presentation, a gene expression profile was not performed. | |

Figure 1
Color photographs and ultrasound biomicroscopy of the anterior segment documenting rapid growth of uveal melanoma involving the iris and ciliary body at initial diagnosis (A) and 6 weeks later, just prior to enucleation (B).
|
|
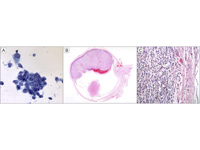
Figure 2
Histopathological analysis of the uveal melanoma. A, Papanicolaou staining shows malignant epithelioid-like cells, some with cytoplasmic pigment, in the aqueous humor, after fine-needle aspiration biopsy. B-C, Hematoxylin-eosin staining showing the enucleated globe with a large melanoma replacing the iris and filling the anterior chamber and almost half of the globe and discontinuous invasion next to the Ahmed valve.
|
|
| Discussion | Noteworthy aspects of this case include the occult nature of ciliary body melanoma and the diagnostic challenge when gonioscopic examination of the angle is made impossible by corneal edema or hyphema. In cases where a complete clinical examination is limited, preoperative evaluation with B-scan ultrasonography or ultrasound biomicroscopy must be considered to rule out an intraocular neoplasm. Although there was an alternative explanation for NVG and hyphema in this case, it is important to maintain a broad differential diagnosis where there is rubeosis and secondary IOP elevation. Secondary IOP elevation is present in up to 17% of ciliary body melanomas at the time of diagnosis and most commonly occurs secondary to direct tumor invasion of the anterior chamber angle and pigment dispersion.(6)
A review of the literature yielded 6 previously published cases of inadvertent aqueous tube shunt implantation in eyes with unrecognized intraocular tumors.(7,8) As would be expected, there was an association between tube shunt insertion and extraocular extension. Similarly, in our patient, extraocular tumor deposits in the inner capsule of the Ahmed valve were identified.
Many solid tumors, including adenocarcinoma of the gastrointestinal tract, express high levels of VEGF and are often treated with a combination of systemic anti-VEGF and additional chemotherapeutic agents.(9) Because angiogenesis and VEGF expression is a common pathway in tumor growth, several authors have investigated its potential role in uveal melanoma. Missotten et al examined 74 enucleated eyes with previously untreated uveal melanoma and concluded that aqueous VEGF concentrations were significantly higher in these specimens compared with healthy eyes undergoing cataract surgery.(10) Moreover, VEGF concentration correlated positively with tumor basal diameter and height.
With regard to the effect of anti-VEGF agents on uveal melanoma, Yang et al found a dose-dependent suppression of primary uveal melanoma growth and formation of hepatic metastases following intraperitoneal injections of bevacizumab in mice.(11) In contrast, when bevacizumab was injected into murine eyes with uveal melanoma, tumor growth accelerated.(12) Lima et al published a series of 3 patients with choroidal melanoma who were initially misdiagnosed with a choroidal neovascular membrane and subsequently received multiple intravitreal bevacizumab injections.(13) There was improvement in subretinal fluid; however, tumor growth continued until choroidal melanoma was diagnosed. Recently, Francis et al conducted a prospective study on 2 eyes with large uveal melanomas (>10 mm in diameter) to evaluate the potential role for neoadjuvant intravitreal bevacizumab.(14) Unfortunately, both patients demonstrated growth at 1 week following injection and underwent enucleation. Lee et al also observed a case of choroidal melanoma demonstrating no growth during a 4.5-month observation period but accelerated growth, from 28.98 mm3 to 66.29 mm3, after 2 intravitreal bevacizumab injections over a 5-month period for persistent subfoveal fluid.(15) Similarly, Mahdjoubi et al found that intravitreal bevacizumab did not reduce the overall enucleation rate in eyes presenting with NVG.(16)
From published estimates, the doubling time of primary uveal melanoma ranges from 23 days to 135 months, with a median of 154-511 days.(4,5,17) In a series of 17 patients, only 2 tumors had doubling times of <40 days.(4) Similarly, Char et al found a median tumor doubling time of 1.4 years, with only 3 cases exhibiting a doubling time of <46 days in a series of 145 choroidal and ciliary body melanomas.(17) Given these trends, our case adds to the prior literature, which suggests a possible link between intravitreal bevacizumab and accelerated primary untreated uveal melanoma growth.
Although several factors may have contributed to our patient’s rapid melanoma growth, we postulate that exposure to bevacizumab played an important role in this cascade. Francis et al hypothesized that certain splice variants of endogenous VEGF actually inhibit angiogenesis.(14) Correspondingly, the inhibition of these endogenous VEGF variants by anti-VEGF therapy may result in “paradoxical” growth of uveal melanoma.(14) In addition, melanoma cells may display vasculogenic mimicry, whereby they form vascular-like channels to provide blood supply to the tumor; however, because these are not true endothelial-lined vessels, they do not respond to anti-angiogenic therapies.(14) Given that our patient received only a single dose of intravitreal anti-VEGF, it is also theoretically possible that a rebound effect following the diminution of exogenous anti-VEGF concentration resulted in accelerated tumor growth. It is also possible, though perhaps less likely, that surgical manipulation during Ahmed valve implantation accelerated tumor growth. Finally, it is important to note that both surgical manipulation of untreated tumor and implantation of an Ahmed valve theoretically contribute to the risk of local and systemic tumor dissemination.
This case highlights the occult nature of ciliary body melanoma and the importance of maintaining a high index of suspicion for this malignancy, despite the presence of other explanations for rubeotic glaucoma. Furthermore, this case adds to the limited body of literature relating anti-VEGF injections and tumor progression in untreated cases of primary uveal melanoma.
Acknowledgments
The authors thank Donna Bong, Ophthalmic Technician at the Royal Alexandra Hospital, for assistance with image acquisition. | | | References | 1. Shields C, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol 2009;127:989-98.
2. Amin M, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017.
3. Corrêa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am J Ophthalmol 2016;162:20-7.
4. Augsburger JJ, Gonder JR, Amsel J, et al. Growth rates and doubling times of posterior uveal melanomas. Ophthalmology 1984;91:1709-15.
5. Gass JDM. Comparison of uveal melanoma growth rates with mitotic index and mortality. Arch Ophthalmol 1985;103:924-31.
6. Shields CL, Shields JA, Shields MB, Augsburger JJ. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology 1987;94:839-46.
7. Kaliki S, Eagle R, Grossniklaus H, Campbell RJ, Shields CL, Shields JA. Inadvertent implantation of aqueous tube shunts in glaucomatous eyes with unrecognized intraocular neoplasms: report of 5 cases. JAMA Ophthalmol 2013;131:925-8.
8. Kiratli H, Koç ?, Tarlan B. Orbital extension of an unsuspected choroidal melanoma presumably through an aqueous tube shunt. Ocul Oncol Pathol 2015;2:144-7.
9. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
10. Missotten G, Notting I, Schlingemann R, et al. Vascular endothelial growth factor a in eyes with uveal melanoma. Arch Ophthalmol 2006;124:1428-34.
11. Yang H, Jager M, Grossniklaus H. Bevacizumab suppression of establishment of micrometastases in experimental ocular melanoma. Invest Ophthalmol Vis Sci 2010;51:2835-42.
12. El Filali M, Ly LV, Luyten GPM, et al. Bevacizumab and intraocular tumors: an intriguing paradox. Mol Vis 2012;18:2454-67.
13. Lima B, Schoenfield L, Singh A. The impact of intravitreal bevacizumab therapy on choroidal melanoma. Am J Ophthalmol 2011;151:323-8.
14. Francis J, Kim J, Lin A, Folberg R, Iyer S, Abramson DH. Growth of uveal melanoma following intravitreal bevacizumab. Ocul Oncol Pathol 2016;3:117-21.
15. Lee J, Kwon H, Kim M, Lee CS, Lee SC. Treatment response to intravitreal bevacizumab in small pigmented choroidal lesions with subretinal fluid. BMC Ophthalmol 2019;19:103.
16. Mahdjoubi A, Najean M, Lemaitre S, et al. Invtravitreal bevacizumab for neovascular glaucoma in uveal melanoma treated by proton beam therapy. Graefes Arch Clin Exp Ophthalmol 2018;256:411-20.
17. Char D, Kroll S, Phillips TL. Uveal melanoma: growth rate and prognosis. Arch Ophthalmol 1997;115:1014-8. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in