|
|
 |
 |
 |
 |
|
|
Optical coherence tomography angiography–guided photodynamic therapy for extrafoveal choroidal neovascularization
Digital Journal of Ophthalmology
2020
Volume 26, Number 1
March 31, 2020
|
Printer Friendly
|
|
|



Rohan Chawla, MD | Dr Rajendra Prasad Center for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India Nasiq Hasan, MD | Dr Rajendra Prasad Center for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India Dheepak Sundar, MD | Dr Rajendra Prasad Center for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India Anu Sharma, MOptom | Dr Rajendra Prasad Center for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India
|
|
|
| Abstract | | Type 1 extrafoveal choroidal neovascularization (CNV) secondary to neovascular age-related macular degeneration was diagnosed in a 68-year-old woman using optical coherence tomography angiography (OCT-A) alone. The entire network of vessels was clearly visible on a 12 × 12 mm OCT-A scan segmented below the retinal pigment epithelium. The patient was initially treated with intravitreal ranibizumab followed by photodynamic therapy (PDT) guided by OCT-A. Complete resolution of subretinal fluid with shrinkage of the neovascular complex was noted 1 month after PDT. | | | Introduction | Neovascular age-related macular degeneration (AMD) is a leading causes of blindness in the elderly worldwide.(1) Intravitreal antivascular endothelial growth factor (anti-VEGF) is the primary treatment for choroidal neovascularization (CNV) in cases of neovascular AMD, followed by photodynamic therapy (PDT);(2) combined anti-VEGF and PDT has also proved successful in many cases.(3) We present a case of extrafoveal CNV treated with ranibizumab and PDT. OCT angiography (OCT-A) alone was used to determine the greatest linear dimension (GLD) of the lesion for determining the spot size to be used for PDT.
| | | Case Report | A 68-year-old woman presented to the retina clinic of Dr Rajendra Prasad Centre for Ophthalmic Sciences, New Delhi, with sudden-onset decrease in vision and metamorphopsia in her right eye of 3 days’ duration. Five years earlier, low vision was noted in the left eye due to a disciform scar following neovascular AMD. On examination, her best-corrected visual acuity was 20/80 in the right eye and 20/1200 in the left eye. Anterior segment examination revealed immature, senile cataract with nuclear sclerosis (grade 2) in both eyes; posterior segment examination of the right eye showed a hypopigmented lesion superotemporal to the fovea with a small area of subretinal blood superior to the lesion (Figure 1A). OCT revealed that the retinal pigment epithelium (RPE) was lifted, with sub-RPE hyperreflectivity and a neurosensory detachment, suggestive of type 1 CNV (Figure 1B). OCT-A segmented below the RPE revealed an area of hyperflow one disc diameter superotemporal to the fovea corresponding to the CNV (Figure 1D). The lesion was better delineated on the larger 12 × 12 mm scan (Figure 1D) than on the routine 4.5 × 4.5 mm OCT-A scan (Figure 1C). The patient was diagnosed with neovascular AMD with extrafoveal CNV in the right eye and a disciform scar in the left eye. Intravitreal ranibizumab 0.5 mg was injected in the right eye as first-line therapy. There was reduction in the subretinal hemorrhage (Figure 2A) and slight flattening of the RPE after the injection; however, subretinal fluid persisted in the extrafoveal region (Figure 2B). Because the CNV was predominantly extrafoveal, PDT was considered.
OCT-A was used to calculate the size of the lesion. The GLD measured was 5328 µm, and a spot size of 6400 µm was used for PDT (Figure 2C). Half fluence PDT was subsequently performed (25 mJ/cm2 for 83 sec). One month following PDT, complete resolution of subretinal fluid was seen on OCT (Figure 2D), and the size of the CNV on OCT-A had considerably reduced (Figure 2E).
The patient was examined monthly after PDT for 4 months. Best-corrected visual acuity in the right eye improved by two lines (20/40), and there was no recurrence of subretinal fluid on OCT. The height of the type 1 CNV reduced on OCT and further shrinking of the CNV complex on OCT-A was noted. No further treatment was done and the patient is on observation.
| |
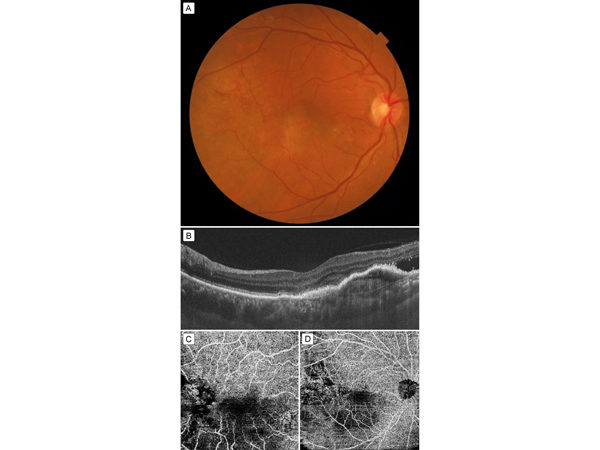
Figure 1
A, Fundus photograph showing a hypopigmented lesion superotemporal to the fovea, with subretinal bleed. B, Swept source OCT (SS-OCT) showing hyperreflective membrane below the retinal pigment epithelium (RPE), or type 1 choroidal neovascularization (CNV), with subretinal fluid. C, 4.5 × 4.5 mm optical coherence tomography–angiography (OCT-A) scan: part of the neovascular component of CNV is seen superotemporal to fovea; however, the entire lesion is not imaged well. D, 12 × 12 mm OCT-A showing the entire neovascular network of the neovascular complex superotemporal to fovea. The scan has been segmented below the RPE.
|
|
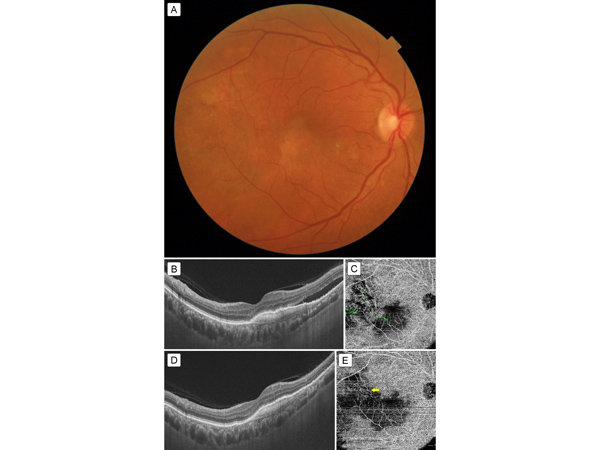
Figure 2
A, Fundus photograph after intravitreal ranibizumab injection showing resolution of the bleed. B, SS-OCT 1 month after injection showing decrease in height of CNV membrane but persistence of subretinal fluid. C, 12 × 12 mm OCT-A scan after injection shows the neovascular network. The greatest linear dimensions of the network are measured to calculate the PDT spot size. D, SS-OCT 1 month after PDT showing complete resolution of subretinal fluid. E, OCT-A 1 month after PDT showing significant shrinkage of neovascular network (yellow arrow).
|
|
| Discussion | PDT was the primary treatment for subfoveal and juxtafoveal CNV before the advent of anti-VEGF agents. However, though progression of CNV could be halted by PDT, there was no real gain in vision. In the present era anti-VEGF agents are the preferred treatment modality for CNV. PDT has been used as an adjunct in cases of polypoidal CNV membranes and type 1 CNV.(4)
PDT uses a diode laser of 689 nm wavelength for 83 seconds. Reduced fluence PDT has become more common in practice, because it decreases the incidence of choroidal ischemia.(5) Commonly, fluorescein angiography or indocyanine green angiography are used to measure the GLD of the lesion, and the laser spot size is set as 1 mm more than the GLD.
OCT-A is a noninvasive imaging modality that can also be used to delineate the CNV network. Depending on the size and location of the lesion, 3 × 3 mm to 12 × 12 mm scans can be performed with the current technology. Because our patient’s CNV was large and extrafoveal, we preferred the 12 × 12 mm scan. We could determine the GLD of the abnormal hyperflow CNV lesion from the scan segmented through it. OCT-A was also helpful in follow-up to demonstrate a reduction in CNV size 1 month after PDT.(6) On follow-up examination, we could clearly identify an extrafoveal CNV with OCT-A. Combination therapy was planned, because we could spare the fovea from the PDT spot. Had there been a recurrence of subretinal fluid despite PDT, we would have continued anti-VEGF therapy. But this combination of PDT and anti-VEGF therapy seemed to work better in this case, at least through 4 months’ follow-up.
To our knowledge, this is the first report of a case in which OCT-A guided PDT has been used to treat neovascular AMD. OCT and OCT-A were sufficient to diagnose, treat, and follow this case. OCT-A is both noninvasive and less time consuming than traditional angiographic methods. It can also be used to plan therapy with anti-VEGF agents and PDT and to assist in confirmation of CNV shrinkage during follow-up. Fluorescein angiography, apart from being invasive and causing minor side effects, including nausea, thrombophelibitis, and, rarely, anaphylaxis, also shows leakage with fuzzy margins of the CNV, which can lead to overestimation of the GLD. Studies have been reported to examine, classify, and treat CNVs with different modalities but the measurement of GLD has not been compared between OCT-A and fluorescein angiography. Larger studies could be useful in comparing fluorescein angiography and indocyanine green angiography with PDT based on OCT-A.
Literature Search
PubMed was searched on February 20, 2020, for English-language results using the following terms: OCT angiography guided photodynamic therapy, photodynamic therapy in choroidal neovascularization, and greatest linear dimension using OCT angiography.
| | | References | 1. Quillen DA. Common causes of vision loss in elderly patients. Am Fam Physician 1999;60:99-108.
2. Cheung GCM, Lai TYY, Gomi F, Ruamviboonsuk P, Koh A, Lee WK. Anti-VEGF Therapy for neovascular AMD and polypoidal choroidal vasculopathy. Asia Pac J Ophthalmol 2017;6:527-34.
3. Agarwal A, Aggarwal K, Gupta V. Management of neovascular age-related macular degeneration: a review on landmark randomized controlled trials. Middle East Afr J Ophthalmol 2016;23:27-37.
4. Otani A, Sasahara M, Yodoi Y, et al. Indocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2007;144:7-14.
5. Reibaldi M, Cardascia N, Longo A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol 2010;149:307-15.
6. Farecki ML, Gutfleisch M, Faatz H, et al. Characteristics of type 1 and 2 CNV in exudative AMD in OCT-angiography. Graefes Arch Clin Exp Ophthalmol 2017;255:913-21.
| |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in