|
|
 |
 |
 |
 |
|
|
Endothelial dysfunction in a child with Pearson marrow-pancreas syndrome managed with Descemet stripping automated endothelial keratoplasty using a suture pull-through technique
Digital Journal of Ophthalmology
2019
Volume 25, Number 4
November 17, 2019
DOI: 10.5693/djo.02.2019.09.001
|
Printer Friendly
Download PDF |
|
|


 Raghav Vadhul, BS
Raghav Vadhul, BS | Indiana University School of Medicine, Indianapolis Caroline S. Halbach, MD | Department of Ophthalmology and Visual Neurosciences, University of Minnesota, Minneapolis Raymond G. Areaux Jr., MD | Department of Ophthalmology and Visual Neurosciences, University of Minnesota, Minneapolis Susan Berry, MD | Department of Pediatrics, University of Minnesota, Minneapolis Joshua H. Hou, MD | Department of Ophthalmology and Visual Neurosciences, University of Minnesota, Minneapolis
|
|
|
| Abstract | | A 4-year-old girl with a history of Pearson marrow-pancreas syndrome presenting with severe, progressive photophobia was found to have bilateral, diffuse corneal thickening and peripheral pigmentary retinopathy. She underwent Descemet stripping automated endothelial keratoplasty (DSAEK) surgery in both eyes using a modified suture pull-through technique. Postoperatively there was no evidence of cataract formation or graft detachment; her corneas thinned, and her photophobia improved dramatically. | | | Introduction | Mitochondrial dysfunction in the cornea can lead to a syndrome similar to Fuchs endothelial dystrophy, wherein excess fluid accumulation causes photophobia and a decrease in visual acuity.(1) This corneal edema is hypothesized to occur because the ion pumps in the endothelial cell layer fail.(2) The pumps depend on primary active transport to maintain fluid balance in the cornea,(3) and mitochondrial dysfunction depletes their energy source and leads to the corneal fluid imbalance observed in these diseases. Other possible explanations for the corneal edema include excess lactate accumulation in the cornea due to compensatory anaerobic glycolysis and increased apoptosis of endothelial cells due to mitochondrial dysfunction.(1)
Pearson marrow-pancreas syndrome is a mitochondrial deletion syndrome that typically arises from a de novo mutation in the mitochondrial DNA. The deletion results in impaired oxidative phosphorylation and commonly involves the bone marrow and the pancreas.(4) Half of the patients with Pearson marrow-pancreas syndrome do not survive past early infancy.(4) About 1 in 3 patients who survive past childhood progressively develop phenotypic drift toward Kearns-Sayre syndrome,(4) which is characterized by progressive ophthalmoplegia, pigmentary retinopathy, cardiac conduction defects, and other systemic symptoms. Corneal endothelial dysfunction has rarely been observed in patients with Pearson marrow-pancreas syndrome, in part because of the high rates of early childhood mortality.(2)
When ocular symptoms from corneal edema start to affect a patient’s daily activities, treatment is indicated.(5) Descemet stripping automated endothelial keratoplasty (DSAEK) is commonly used for definitive treatment of corneal edema in pediatric patients. However, the procedure requires supine positioning for the first 24 hours after surgery and thus presents a challenge for children.(5) We report a rare case of Pearson marrow-pancreas syndrome with corneal manifestations in a child and the first use of endothelial keratoplasty to treat this disease. We also introduce DSAEK with a modified suture pull-through technique that could improve outcomes in pediatric patients. | | | Case Report | A 4-year-old girl with history of a mitochondrial deletion syndrome was referred to the Cornea Service at the University of Minnesota for evaluation of corneal edema. Starting at age 2 years, she began to develop progressively worsening photophobia, which became so severe that she became house-bound and self-confined to her darkened room. Previous genetic testing performed after she presented emergently for weakness, dehydration, thrombocytopenia, and electrolyte abnormalities revealed a large, heteroplasmic deletion of mitochondrial DNA spanning nucleotides 6249 to 14098. The patient was diagnosed with Pearson marrow-pancreas syndrome with associated Fanconi’s syndrome.
The patient was initially seen by a pediatric ophthalmologist at age 2 years for her ocular symptoms and was noted to have dimmed red reflex, mild corneal haze, and increased central corneal thickness bilaterally. She was started on sodium chloride drops and oral coenzyme Q10 supplements, with minimal improvement in symptoms. By 4 years of age, she was referred for possible endothelial keratoplasty. Examination under anesthesia at that time revealed corneal edema with mild haze but no guttae or endothelial abnormalities bilaterally. Pachymetry was 865 μm in the right eye and 875 μm in the left eye. The patient subsequently underwent bilateral DSAEK using a modified suture pull-through technique. Histology from Descemet’s membrane fragments removed during stripping showed severe loss of endothelial cells but no guttae (Figure 1).
Postoperatively, the patient’s photophobia improved dramatically. By postoperative month 5, pachymetry had improved to 685 μm in the right eye and 755 μm in the left eye (preoperative graft thicknesses were 100 μm in the right eye and 140 μm in the left eye). See Figure 2. Dilated fundus examination showed progressive peripheral pigmentary retinopathy bilaterally (Figure 3). Electroretinogram testing showed severe dysfunction of rods and cones, without photoreceptor loss. Cardiac evaluation and echocardiography at that time were normal, with no evidence of conduction abnormalities. At last follow-up, at age 7 years, the patient had no photophobia, was correctable to 20/50 in each eye, had clear corneas, clear lenses, mild progression of peripheral pigmentary retinopathy, and a small, intermittent left exotropia.
Modified DSAEK
Because of this patient’s phakic status and questionable ability to position supine after surgery, her DSAEK procedures were performed using a modified suture pull-through technique (Figure 4). For both surgeries, Descemet’s membrane was stripped under viscoelastic. An anterior chamber maintainer was then inserted through a 20-gauge paracentesis incision and irrigation and aspiration were performed to remove residual viscoelastic. A precut donor cornea was trephinated to 8.0 mm, and a small amount of viscoelastic was placed over the endothelium before the graft was carefully folded over into a 50-50 taco. Under the surgical microscope, a double-armed, 10-0 polypropylene suture on a CTC6 needle was used to imbricate the graft using a partial-thickness pass through the posterior fold of the graft, approximately 0.5 mm from the edge (Figure 4).
The superior corneal incision was then widened to 4.25 mm. Under continuous irrigation from the anterior chamber maintainer, both arms of the suture were passed through the corneal incision and out the inferior cornea at the margins of the stripping. Care was taken to ensure that the suture arms did not cross and were oriented in such a manner that the stromal side of the graft would be pulled against the recipient cornea (Figure 5). The graft was then pulled into the eye by drawing on both arms of the suture. The main corneal incision was then sutured closed. Gentle sweeps across the cornea were used to refine the centration. After removing the anterior chamber maintainer and achieving 100% air fill for 5 minutes, the polypropylene suture was tied in a loose air knot and left in place to support the inferior graft. The polypropylene suture was removed under sedation on postoperative day 1. Care was taken to avoid overtightening the knot and creating striae. The air fill was then titrated to 60%-70%, and the pupil dilated with atropine ophthalmic 1% to prevent pupillary block. | |
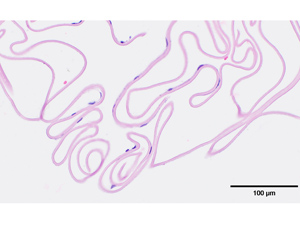
Figure 1
Stripped Descemet membrane showing severe endothelial cell loss without guttae (hematoxylin-eosin, original magnification ×40).
|
|
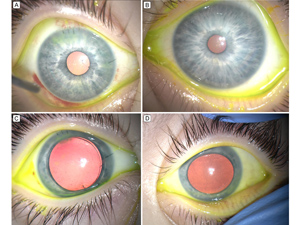
Figure 2
Intraoperative photographs of the right eye after corneal transplantation (A, C) and the left eye before corneal transplantation (B, D). Significant corneal clouding is evident in the untreated left eye, but not in the right eye following Descemet stripping automated endothelial keratoplasty (DSAEK).
|
|
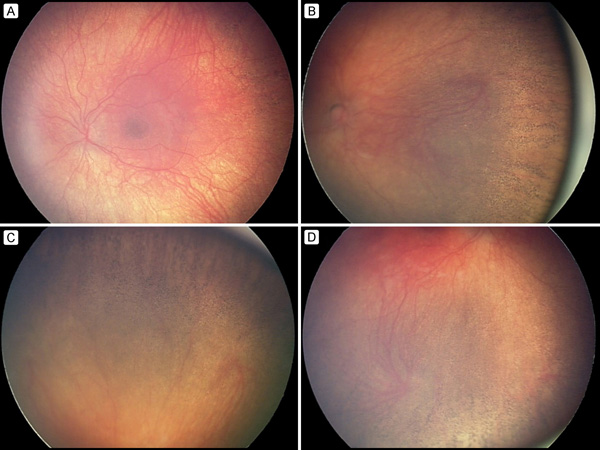
Figure 3
RetCam photographs of the left eye (A, C) and right eye (B, D) showing pigmentary retinopathy in the peripheral retina.
|
|
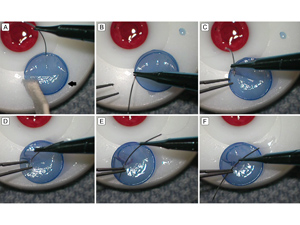
Figure 4
Intraoperative photographs showing the technique for imbricating the donor graft. A, After placing viscoelastic over the endothelial surface, the DSAEK graft is gently folded over into a 50-50 taco on the stromal cap (arrow). B-C, While choking up on the needle for improved control, one arm of a double armed 10-0 polypropylene is driven through the posterior fold of the DSAEK graft approximately 0.5 mm from the edge of the graft. Care is taken to obtain only a partial-thickness bite that does not violate endothelium. D-F, The needle is passed completely through the graft using a pair of 0.12 forceps to gently brace the graft at the needle exit site.
|
|

Figure 5
Intraoperative photographs demonstrating the modified suture pull-through technique (superior is located at 6:00 in the images). A-B, After imbricating the donor DSAEK graft with a double-armed, 10-0 polypropylene suture, both arms of the suture are passed through the corneal wound and out through the inferior cornea. C-D, Care is taken to avoid crossing the arms of the suture. E, The graft is pulled into the anterior by drawing on both arms of the suture. Once in the anterior chamber, the suture helps maintain the proper orientation and centration of the graft. F, The main wound is then closed with 10-0 nylon suture and air is injected into the anterior chamber to lift the DSAEK up against the recipient cornea. The polypropylene suture is then tied in a loose air knot and left in place.
|
|
| Discussion | Pearson marrow-pancreas syndrome is a mitochondrial deletion syndrome associated with sideroblastic anemia and exocrine pancreatic dysfunction. Progressive onset of ocular symptoms mimicking Kearn-Sayre, including pigmentary retinopathy, external ophthalmoplegia, and corneal edema has been described.(2) However, ocular manifestations of the disease remain poorly characterized. We describe early-onset of corneal edema and pigmentary retinopathy in Pearson marrow-pancreas syndrome associated with severe photophobia. Previous descriptions of corneal edema in Kearn-Sayre have been associated with guttae,(6) but that was not observed clinically or histologically in our case. Loss of endothelial cells on histological sample of Descemet’s membrane was noted but may have been due to traumatic stripping. Although coenzyme Q10 has been reported to be effective in corneal edema associated with Kearn-Sayre,(6) treatment was ineffective in our patient. The patient’s corneal edema and photophobia did, however, respond well to DSAEK bilaterally. Intraocular pressure at the most recent office visit were 18 mm Hg in the right eye and 19 mm Hg in the left eye. She was maintained on loteprednol 1% ophthalmic drops administered bilaterally every other day.
Endothelial keratoplasty is replacing penetrating keratoplasty (PKP) as the preferred treatment of endothelial failure in children because of intra- and postoperative risks associated with pediatric PKP.(7,8) However, like PKP, DSAEK is more challenging to perform in children than in adults. Postoperatively, poor compliance with supine positioning increases the risk of graft detachment.(8,9) Intraoperatively, shallow anterior chamber, frequent anterior chamber collapse, and iris prolapse make graft insertion difficult.(8) Furthermore, risk of lenticular trauma remains a critical concern since most pediatric patients are phakic.(9-12) Modified techniques for DSAEK insertion, for example, using a sheets glide to protect the lens, have been proposed to minimize lenticular trauma.(12) However, techniques for pediatric DSAEK continue to evolve.
The modified suture pull-through technique we describe both avoids lenticular trauma and facilitates placement of an inferior stay suture to ensure graft attachment. Unlike previously described suture pull-through techniques, where a single-armed suture is used,(13,14) our technique uses a double-armed suture. The anterior chamber remains formed under continuous infusion throughout surgery, minimizing the risk to the lens. After attachment of the graft, the suture arms are tied to create a stay suture that supports adhesion of the inferior graft. This allows patients to be mostly upright, with intracameral air mainly supporting the superior graft. In pediatric patients who may have difficulty staying supine postoperatively, this may be particularly advantageous.
Literature Search
PubMed was searched on May 3, 2019, for English-language results, using the following terms and combinations: Pearson syndrome AND cornea, (DSAEK OR Descemet stripping automated endothelial keratoplasty) AND endothelial dysfunction, corneal transplant AND pediatric, and (DSAEK OR Descemet stripping automated endothelial keratoplasty) AND suture pull through. Search results published over 11 years ago were excluded. Type of study was not specified in our search criteria. Additional studies were found by reviewing the articles cited within the search results. | | | References | 1. Boonstra F, Claerhout I, Hol F, Smit G, van Collenburg J, Meire F. Corneal decompensation in a boy with Kearns-Sayre syndrome. Ophthalmic Genet 2000;23:247-51.
2. Kasbekar S, Gonzalez-Martin J, Shafiq A, Chandna A, Willoughby CE. Corneal endothelial dysfunction in Pearson syndrome. Ophthalmic Genet 2013;34:55-7.
3. Bonanno J. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res 2012;95:2-7.
4. Manea E, Leverger G, Bellmann F, et al. Pearson syndrome in the neonatal period: two case reports and review of the literature. J Pediatr Hematol Oncol 2009;31:947-51.
5. Mau K. What DSAEK is going on? An alternative to penetrating keratoplasty for endothelial dysfunction. Optom J Am Optom Assoc 2009;80:513-23.
6. Kim J, Medsinge A, Chauhan B, et al. Coenzyme Q10 in the treatment of corneal edema in Kearns-Sayre. Cornea 2016;35:1250-4.
7. Zhu A, Marquezan M, Kraus C, Prescott C. Pediatric corneal transplants. Cornea 2018;37:973-80.
8. Trief D, Marquezan M, Rapuano C, Prescott C. Pediatric corneal transplants. Curr Opin Ophthalmol 2017;28:477-84.
9. Mittal V, Mittal R. Challenges in pediatric endothelial keratoplasty. Indian J Ophthalmol 2014;62:251.
10. Madi S, Santorum P, Busin M. Descemet stripping automated endothelial keratoplasty in pediatric age group. Saudi J Ophthalmol 2012;26:309-13.
11. Kymionis G, Kankariya V, Diakonis V, Karavitaki AE, Siganos CS, Pallikaris IG. Descemet stripping automated endothelial keratoplasty in a child after failed penetrating keratoplasty. J AAPOS 2012;16:95-6.
12. Panahi-Bazaz M, Sharifipour F, Malekahmadi M. Modified Descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. J Ophthalmic Vis Res 2013;9:522-5.
13. Sarnicola V, Toro P. Descemet-stripping automated endothelial keratoplasty by using suture for donor insertion. Cornea 2008;27:825-9.
14. Sarnicola V, Millacci C, Sarnicola E, Sarnicola C, Sabatino F, Ruggiero A. Suture pull-through insertion of graft donor in Descemet stripping automated endothelial keratoplasty: results of 4-year follow-up. Taiwan J Ophthalmol 2015;5:114-9.
| |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in