|
|
 |
 |
 |
 |
|
|
Advanced spheroidal degeneration
Digital Journal of Ophthalmology
2019
Volume 25, Number 4
December 22, 2019
|
Printer Friendly
Download PDF |
|
|



Abdelrahman M. Elhusseiny, MD | Department of Ophthalmology, Kasr Al-Ainy Hospitals, Cairo University, Giza, Egypt Reem H. El Sheikh, MD | Department of Ophthalmology, Kasr Al-Ainy Hospitals, Cairo University, Giza, Egypt Emery Jamerson | Department of Ophthalmology, Columbia University Medical Center, New York Islam Y. Swaify, MD | Department of Ophthalmology, Kasr Al-Ainy Hospitals, Cairo University, Giza, Egypt Ahmed B. Araissi, MD | Department of Ophthalmology, Kasr Al-Ainy Hospitals, Cairo University, Giza, Egypt Abdelaziz A. Saad, PhD | Department of Ophthalmology, Kasr Al-Ainy Hospitals, Cairo University, Giza, Egypt
|
|
|
| Abstract | | Spheroidal degeneration, involving the cornea and/or the conjunctiva, is characterized by amber-colored homogeneous, translucent spherules in the corneal stroma, Bowman’s membrane, and subepithelium. The condition has a higher prevalence in areas with extreme temperatures, low humidity, high wind, and presence of sand. We report the case of a 46-year-old man with a 10-year history of gradual progressive diminution of vision, severe blepharospasm, and photophobia. Examination revealed bilateral plaques of amber-colored nodules covering about half of the cornea. Superficial keratectomy was performed for the lesions in both eyes, leaving an epithelial defect overlying a plane of opaque cornea. Histopathology showed amorphous protein in the anterior stroma, confirming the clinical diagnosis of advanced grade 4 spheroidal degeneration. Visual acuity and other symptoms dramatically improved, and the patient was scheduled for keratoplasty. | | | Introduction | Spheroidal degeneration, also known as Labrador keratopathy, Fisherman’s keratopathy, climatic droplet keratopathy, actinic keratopathy, and Bietti’s band-shaped nodular dystrophy, is a degeneration of the cornea and/or the conjunctiva that is characterized by amber-colored homogeneous, translucent spherules of varying sizes in the corneal stroma, Bowman’s membrane, and subepithelium. These usually bilateral, amber-colored granules appear at peripheral cornea, coalesce, becoming nodular, and spread more to involve central cornea.(1,2)
A higher prevalence of spheroidal degeneration has been noted in areas with extreme temperatures, low humidity, high wind, presence of sand, and high levels of exposure to ultraviolet radiation; prior studies have estimated the prevalence from 6% in London to 18% in the United States to 57% in Ethiopia.(3,4) It has also been noted to have a male predominance (male-to-female ratio of 7:3).(4) Multiple, pathologically indistinguishable subclassifications of spheroidal degeneration have been documented: primary spheroidal degeneration is attributed to the aforementioned risk factors and not associated with other ocular pathology; secondary spheroidal degeneration is thought to occur secondary to other ocular inflammation and corneal pathology, and tertiary spheroidal degeneration is associated with conjunctival findings and more frequently associated with pinguecula.(5)
Welding has also been shown to be associated with spheroidal degeneration.(6) The exact molecular mechanism underlying the formation of spheroidal degeneration has yet to be elucidated, but prior studies have suggested that albumin and immunoglobulins diffuse from the limbal circulation into the cornea and are modified by UV radiation.(7)
Clinically, spheroidal degeneration can be classified according to Johnson and Ghosh system into four grades: grade 1, with lesions involving the interpalpebral cornea but not the central cornea; grade 2, involving the central cornea but not affecting visual acuity; grade 3, with central corneal involvement associated with a decline in visual acuity; and grade 4, with elevated lesions.(8) | | | Case Report | A 46-year-old white man from Upper Egypt presented with a 10-year history of bilateral gradual progressive diminution of vision associated with foreign body sensation, photophobia, constant lacrimation, and eyelid edema. He was a farmer, who worked outdoors more than 10 hours daily for more than 30 years. The patient reported no family history of a similar ocular condition. Ophthalmological examination disclosed bilateral diffusely swollen upper eyelids with soft, nontender edema and severe blepharospasm (Figure 1).
A gush of tears was expressed when the eyelids were opened. Anterior segment examination showed a plaque of amber-colored elevated nodules present on the anterior surface of both corneas, extending from limbus to limbus and vertically covering the inferior half of the cornea; the affected area measured about 11 × 5 mm on each side (Figure 2). The upper half of the cornea was hazy, and the rest of the anterior segment could not be evaluated. Visual acuity in each eye was hand motions, with good perception and projection of light. Findings were bilateral and symmetrical (Figure 2). Anterior segment optical coherence tomography could not be performed because of the severe photophobia.
The patient underwent superficial keratectomy for the right eye; the excisional biopsy was submitted for histopathology. The lesion was well defined and could be easily excised as one mass, leaving an epithelial defect overlying a plane of opaque cornea postoperatively (Figure 3). He underwent the same procedure for the left eye 1 week later. A few days postoperatively the epithelial defect healed, with leukoma, in both eyes. Visual acuity improved to 1/60 in each eye, with marked improvement in the patient’s symptoms; the patient was able to open both eyes, with lessening of foreign body sensation. Lid edema dramatically regressed within the first 2 weeks postoperatively. The patient was scheduled for bilateral keratoplasty.
Histopathological examination of the lesion (hematoxylin and eosin staining) revealed amorphous globules of protein deposits in the anterior stroma (Figure 4) that correlated with the clinical diagnosis of grade 4 spheroidal degeneration.(8) Congo red stain for amyloid was negative excluding gelatinous droplike corneal dystrophy as a possible alternative diagnosis.
The patient was followed regularly for 6 months, during which time his condition remained stationary. He eventually sought treatment elsewhere, because of the long waiting list for keratoplasty at our hospital. | |

Figure 1
Lateral external photograph highlighting the severity of the upper eyelid edema.
|
|
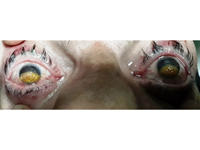
Figure 2
External photograph showing bilateral symmetric plaques of amber-colored elevated nodules present on the surface of both corneas measuring 11 × 5 mm.
|
|

Figure 3
Photograph of the right eye at the end of the surgery showing inferior epithelial defect, overlying a plane of corneal opacity, that remained after removal of the lesion in one piece.
|
|
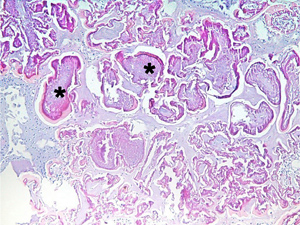
Figure 4
Histopathological section of the lesion showing amorphous protein globules (stars) within the anterior stroma on routine staining (hematoxylin-eosin, original magnification ×10).
|
|
| Discussion | Spheroidal degeneration is a degenerative corneal condition characterized by homogenous, amber-colored spherules at or beneath the corneal or conjunctival epithelium. These spherules have histological similarities to elastic fibers of pingueculae, suggesting the presence of degenerate collagen, but differences between the two suggest the presence of an additional noncollagenous protein as well.(9)
The findings obtained by histopathological staining of corneal tissues in spheroidal degeneration are consistent.(4) Immunoperoxidase staining has located droplets in areas with the greatest concentration of plasma proteins, such as albumin and immunoglobulins G and A, supporting the postulate that additional noncollagenous proteins are implicated in the disease pathophysiology.(10)
Given the patient’s initial presentation, spheroidal degeneration, Salzmann’s nodular degeneration, Vogt limbal degeneration and gelatinous droplike corneal dystrophy were possible causes for the patient’s symptoms and corneal findings. The lack of symptoms until the middle of the third decade of life as well as the patient’s occupation as a farmer favored a clinical diagnosis of spheroidal degeneration, confirmed by the presence of amorphous protein globules detected on histopathological examination of the excisional biopsy. Negative Congo red reaction with the absence of apple-green birefringence under polarized light confirmed the absence of amyloid proteins and excluded the possibility of gelatinous droplike corneal dystrophy.
In contrast to previously reported cases of spheroidal degeneration, in our patient the lesions were extensive, and the symptoms were severe. The majority of patients with spheroidal degeneration are asymptomatic, and visual impairment is uncommon but can occur as opacities accumulate at the central cornea (grades 3 and 4).(8) Our patient, however, presented with bilateral symptoms of progressive vision loss to hand motions, eyelid swelling, foreign body sensation, photophobia and lacrimation for over a decade—symptoms vastly more significant and impairing than those previously documented in the literature. In fact, the patient’s photophobia prevented his completion of additional imaging studies, including anterior segment OCT. The patient also had hazy corneal opacities superior to the amber meridian created by the spheroidal degeneration that prevented further visualization of intraocular structures.
As lesions become more elevated in classically described spheroidal degeneration, epithelial defects, recurrent erosions, and sterile ulceration can occur.(1) In our case, however, the lesion was able to be excised while leaving the entirety of the remaining cornea intact; this outcome is consistent with reported cases, which describe the spheroidal degeneration as being located in the superficial layers of the cornea. The patient did not develope any epithelial defects, erosions, or ulcerations, however, until after excision of the corneal mass. The diagnosis of spheroidal degeneration is largely clinical, but biopsy and histological staining techniques are helpful in its confirmation. The protein deposits in anterior stroma visualized on histopathology are consistent with this diagnosis as well.
There is currently no medical treatment for spheroidal degeneration. Patients with mild-to-moderate, visually significant disease can be treated surgically with superficial keratectomy or phototherapeutic keratectomy, whereas patients with advanced disease require corneal transplantation. Due to the rarity of spheroidal degeneration progression to such a degree, there is limited data on post-transplant recurrence of spheroidal degeneration. Prior case studies have shown recurrence of spheroidal degeneration after a mean of 4 years, with the disease beginning in the periphery of the graft and moving centrally.(11) These reports include patients who have been continuously exposed to the aforementioned spheroidal degeneration risk factors after keratoplasty. Careful follow-up is needed after keratoplasty, and patients should be counseled to avoid ocular UV radiation exposure, to use protective eyeglasses while working outdoors,(6) advised regarding adequate dietary intake of ascorbic acid to protect against UV radiation-induced oxidative stress.(12-14)
Literature Search
PubMed was searched in September 2018, for English-language results, using the following terms: spheroidal degeneration, Labrador keratopathy, and climatic droplet keratopathy. | | | References | 1. Magovern M, Wright JD, Mohammed A. Spheroidal degeneration of the cornea: a clinicopathologic case report. Cornea 2004;23:84-8.
2. Farjo Q, Sugar A. Corneal degenerations. In: Yanoff M, Duker JS, eds. Ophthalmology 3rd ed. St. Louis: Mosby/Elsevier; 2009:318-23.
3. Mohan A, Jamil Z, Bhatanagar VC, Gajraj M. Prevalence of spheroidal degeneration of cornea and its association with other eye diseases in tribes of Western Rajasthan. Indian J Ophthalmol 2017;65:1010-14.
4. Garner A, Fraunfelder FT, Barras TC, Hinzpeter EN. Spheroidal degeneration of cornea and conjunctiva. Br J Ophthalmol 1976;60:473-8.
5. Hanna C, Fraunfelder FT. Spheroid degeneration of the cornea and conjunctiva. Am J Ophthalmol 1972;74:829-39.
6. Norn M, Franck C. Long-term changes in the outer part of the eye in welders: prevalence of spheroid degeneration, pinguecula, pterygium, and corneal cicatrices. Acta Ophthalmol 1991;69:382-6.
7. Johnson GJ, Overall M. Histology of spheroidal degeneration of the cornea in Labrador. Br J Ophthalmol 1978;62:53-61.
8. Johnson GJ, Ghosh M. Labrador keratopathy: clinical and pathological findings. Can J Ophthalmol 1975;10:119-35.
9. Lai K, Reidy J, Bert B, Milman T. Spheroidal degeneration in H626R TGFBI variant lattice dystrophy: a multimodality analysis. Cornea 2014;33:726-32.
10. Suarez MF, Piqueras MC, Correa L, et al. Phospholipidomic studies in human cornea from climatic droplet keratopathy. J Cell Biochem 2017;118:3920-31.
11. Al-Rajhi AA, Cameron JA. Recurrence of climatic droplet keratopathy: two case reports. Acta Ophthalmol Scand 1996;74:642-4.
12. Serra HM, Suarez MF, Esposito E, Urrets-Zavalia JA. Vitamin C functions in the cornea: ultra-structural features in ascorbate deficiency. In: Preedy VR, ed. The Handbook of Nutrition, Diet and the Eye. London, UK: Elsevier Inc; 2014:311-320.
13. Suárez MF, Correa L, Crim N, et al. Climatic droplet keratopathy in Argentina: involvement of environmental agents in its genesis which would open the prospect for new therapeutic interventions. Biomed Res Int 2015;2015:1-9.
14. Serra HM, Holopainen JM, Beuerman R, Kaarniranta K, Suárez MF, Urrets-Zavalía JA. Climatic droplet keratopathy: an old disease in new clothes. Acta Ophthalmol 2015;93:496-504. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in