|
|
 |
 |
 |
 |
|
|
Band keratopathy developing after denosumab injections
Digital Journal of Ophthalmology
2019
Volume 25, Number 2
June 12, 2019
|
Printer Friendly
Download PDF |
|
|



Michael Trong Duc Nguyen, MD | Département d’ophtalmologie, Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada Mikael Sebag, MD, FRCSC | Département d’ophtalmologie, Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada Mona Harissi-Dagher, MD, FRCSC | Département d’ophtalmologie, Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada
|
|
|
| Abstract | | We report the case of a 75-year-old woman who developed band keratopathy following denosumab therapy. The patient was referred for evaluation of progressive vision loss and new-onset band keratopathy in both eyes following denosumab therapy. She had no prior ocular history. On examination, she had calcific deposits in a horizontal band in the interpalpebral superficial cornea. Laboratory workup was negative. Denosumab was discontinued, and she was treated with keratectomy with ethylene-diamine-tetra-acetic acid. Denosumab influences calcium metabolism and consequently reduces bone turnover and increases bone density. It is commonly used for treatment of osteoporosis at high-risk for fracture. Very few cases of ocular adverse drug reactions have been reported. However, because of temporal association and biological plausibility, we believe our patient developed progressive band keratopathy after administration of denosumab. | | | Case Report | A 75-year-old white woman was referred by her ophthalmologist to the cornea clinic of the Centre Hospitalier de l’Université de Montréal for treatment of band keratopathy in both eyes. She complained of progressive vision loss in both eyes over the course of 2 years. She had no other ocular symptoms, and review of systems was negative. There was no history of other ocular disease and no prior use of eyedrops. Her medical history included postmenopausal osteoporosis, for which she had received four injections of denosumab every 6 months over a period of 2 years. She was also on bisoprolol (Sandoz Bisoprolol, Sandoz Canada Inc, Boucherville, QC) for her hypertension, but she was otherwise in good health. Family history was negative.
Her best-corrected visual acuity was 20/100-2 in the right eye and 20/80 in the left eye. Intraocular pressure was 14 mm Hg in each eye. Slit-lamp examination showed calcific deposits in a horizontal band in the interpalpebral superficial cornea (Figure 1), worse on the left. She had mild nuclear cataracts in each eye. Fundus examination showed a temporal submacular hemorrhage in the right eye, for which she was concurrently followed by a retina specialist, and 3+ macular drusen in the left eye. Cup-to-disc ratio was 0.3 in each eye. Laboratory workup showed normal urinalysis and serum electrolytes, including calcium and phosphate levels. Serum 25(OH)-vitamin D levels were normal.
Denosumab injections were discontinued. A keratectomy with chelating agent ethylene-diamine-tetra-acetic acid 1% was performed in the left eye. At her 1-week follow-up examination, evolution was favorable (Figure 2). Postoperative visual acuity was 20/80-2 in the right eye and 20/70-1 in the left eye (increasing to 20/60 on pinhole). | |

Figure 1
Band keratopathy in the right eye. A, Calcific deposits are seen in Bowman’s layer in the interpalpebral area. B, Retroillumination of the right eye to highlight the deposition in the cornea.
|
|
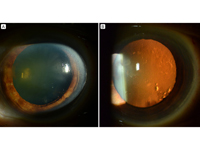
Figure 2
Left eye after surgery. A, Band keratopathy has been removed successfully, and the cornea is clear. B, Retroillumination of the left eye shows no residual deposition in the cornea and only some irregularities related to the lens.
|
|
| Discussion | Denosumab is a human immunoglobulin G2 (IgG2) monoclonal antibody to the receptor activator of nuclear factor kappa-B ligand (RANKL), the main regulator of osteoclastic bone resorption.(1) In osteoporosis, RANKL levels are high. By inhibiting RANKL’s action, denosumab reduces the rate of bone turnover, increasing bone density and reducing the risk of fractures.(1) A review of denosumab randomized controlled trials (RCTs) revealed a safe and tolerable profile, with expected benefits outweighing potential risks.(1) The most common side effects are “back pain, arm and leg pains, high cholesterol, muscle pain, and bladder infections.”(2)
In 2009 the Food and Drug Administration safety analysis of denosumab identified ophthalmic side effects as one of its “adverse events of special interest” because of a discrepancy in the incidence of cataracts in some RCTs.(3) However, ocular adverse reactions from denosumab therapy are unusual. Nonetheless, a case of potential denosumab-related subretinal bone formation was reported after a patient underwent surgical resection of a giant cell tumor of the sphenoid and subsequent denosumab therapy.(4)
In its product monograph, Amgen Canada Inc lists all ocular events from its RCTs, including “cataract, glaucoma, conjunctivitis allergic, dry eye, ocular discomfort, eyelid pain, lacrimation, visual disturbance, vitreous disorder, conjunctivitis, eye pain, arteriosclerotic retinopathy, blepharitis, blepharospasm, eyelids pruritus, lacrimal gland enlargement, photophobia, vision blurred, and vitreous hemorrhage.”(5) Most events occurred in <1% of patients. Band keratopathy is not mentioned. Hypercalcemia, renal impairment, and hyperparathyroidism occurred in <1% of cases.(5) Of note, adverse events with denosumab were not compared to placebo, and no risk ratio was calculated.
We believe that our patient developed band keratopathy as a result of denosumab therapy. Band keratopathy is a calcific degeneration of the superficial cornea, mainly involving Bowman’s layer. Its main known risk factors are chronic ocular disease (usually inflammatory, as in uveitis, multiple ocular surgeries, etc), hypercalcemia, hereditary transmission, hyperphosphatemia with normal calcium levels (usually in renal failure), chronic exposure to mercurial vapors or mercurial preservatives (in some eyedrops), and silicone oil in aphakic eyes.(6)
Except for denosumab therapy, our patient’s history and laboratory workup for band keratopathy did not suggest alternative causes of band keratopathy, including underlying metabolic or renal disease. In our patient, progressive vision loss and band keratopathy developed after initiation of denosumab treatment. Because denosumab inhibits RANKL, which plays a significant role in bone turnover and influences calcium phosphate metabolism, we believe that the drug caused an imbalance in calcium and phosphate levels. For example, undetected or subclinical hypercalcemia may have led to progressive accumulation of calcific deposits in our patient’s cornea. Using Naranjo’s algorithm of adverse drug reaction probability,(7) we obtained a score of 2, which corresponds to a “possible” reaction. Therefore, we suggest considering band keratopathy as a possible adverse reaction to denosumab, because it followed a temporal sequence after administration of the drug, has a biologically plausible association with its influence on calcium metabolism. Further follow-up of the patient and additional reporting of similar cases will help to establish the likelihood of band keratopathy being an adverse reaction to denosumab therapy.
Because denosumab is a fairly new drug, all potential adverse reactions should be reported to the appropriate regulatory authorities to better establish its short- and long-term safety profile. Considering the likelihood of increased usage of denosumab in an aging population, careful surveillance for adverse events with this drug is advisable.
Literature Search
We performed a systematic review of PubMed and EMBASE on July 27, 2018, without language restriction, of case reports presenting band keratopathy after denosumab injections using the following terms: denosumab, band keratopathy, adverse reactions, and side effects. | | | References | 1. Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011;3:79-91.
2. Amgen. Medication Guide: Prolia®. Thousand Oaks, CA: Amgen Inc; 2011. Available from: http://pi.amgen.com/united_states/prolia/prolia_mg.pdf. Accessed July 27, 2018.
3. Rothstein A. Denosumab Safety: FDA Analysis. Available at: http://courses.washington.edu/bonephys/denosumab/Rothstein%20FDA%20deno%20safety.pdf. Accessed September 6, 2011.
4. Hwang CK, Baker Hubbard G, Rapkin L, Grossniklaus HE. Subretinal bone formation in a patient with giant cell tumor of the sphenoid. Ocul Oncol Pathol 2014;1:34-8.
5. Amgen Canada Inc. Prolia (denosumab) Product Monograph. Available at: https://www.amgen.ca/products/~/media/1e79aee7d94340df88c3d97f5bb897c3.ashx. Accessed July 27, 2018.
6. Roy FH . Corneal and conjunctival calcification. In: Roy FH, Fraunfelder FW, Fraunfelder FT. Roy and Fraunfelder’s Current Ocular Therapy. 6th ed. Current Therapy series. Philadelphia: Elsevier/Saunders; 2008:337-8.
7. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
| |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in