|
|
 |
 |
 |
 |
|
|
Diffuse ocular and orbital inflammation after zoledronate infusion—case report and review of the literature
Digital Journal of Ophthalmology
2017
Volume 23, Number 4
December 28, 2017
DOI: 10.5693/djo.02.2017.08.002
|
Printer Friendly
Download PDF |
|
|



Obi C. Umunakwe, MD, PhD | Ophthalmology and Visual Sciences, Vanderbilt University Medical Center, Nashville, Tennessee David Herren, MD | Ophthalmology and Visual Sciences, Vanderbilt University Medical Center, Nashville, Tennessee Stephen J. Kim, MD | Ophthalmology and Visual Sciences, Vanderbilt University Medical Center, Nashville, Tennessee Sahar Kohanim, MD, MPH | Ophthalmology and Visual Sciences, Vanderbilt University Medical Center, Nashville, Tennessee
|
|
|
| Abstract | | Bisphosphonates have become a commonly used class of medications to treat osteoporosis and other bone diseases. Zoledronate (zoledronic acid) can be dosed annually via intravenous infusion, making it an appealing option for patients and physicians. We report the case of a 68-year-old woman who developed severe, unilateral, ocular inflammation, including corneal endotheliitis, anterior uveitis with hyphema, scleritis, and orbital inflammation beginning 12 hours after receiving her first zoledronate infusion. Symptoms escalated but ultimately resolved with topical steroids and high-dose systemic corticosteroids. To our knowledge, this is the first report of unilateral diffuse inflammation of the eye and orbit, including corneal inflammation developing within 12 hours of a first zoledronate infusion. | | | Introduction | | Bisphosphonates have become the most commonly used medication for treatment of osteoporosis.(1) They are also used in conditions such as hypercalcemia, Paget disease of bone, and bony metastatic disease.(2) Zoledronate is among the most frequently used bisphosphonates because it permits an annual dosing regimen via intravenous infusion. Ocular complications related to bisphosphonates have previously been described and are rare. Most of these occurrences are limited to nonspecific conjunctivitis, anterior uveitis, or blurred vision with no long-term sequelae.(3) However, bisphosphonates have also been reported to cause scleritis, their most vision-threatening side effect.(3,4) | | | Case Report | A 68-year-old woman presented at an outside hospital with a 24-hour history of right eye pain, redness, and photophobia. Her past medical history was notable for hypertension, hyperlipidemia, depression/anxiety, migraine headaches, and osteoporosis. Her family history was noncontributory. Symptoms of right eye pain, redness, and photophobia began within 12 hours of her first-ever intravenous infusion of 4 mg of zoledronate to treat osteoporosis. These symptoms were associated with fever, myalgias, and arthralgias. She was evaluated at her local emergency department and treated with a topical antibiotic. The next day, she was examined by an ophthalmologist at the Vanderbilt Eye Institute, who found decreased visual acuity (20/50), conjunctival injection, 4+ cell and 2+ flare in the anterior chamber, layered hypopyon with hyphema, globe tenderness, and active scleritis. A diagnosis of bisphosphonate-associated anterior uveitis and scleritis was presumed, and she was started on topical steroids, cycloplegics, and oral nonselective anti-inflammatory drugs (NSAIDs).
Two days later, she returned with worsening symptoms and was found to have visual acuity of 20/400, conjunctival injection, 3+ to 4+ cell and flare, scleral inflammation, and globe tenderness, with the addition of ptosis, corneal edema, posterior synechiae, and Descemet’s folds. B-scan ultrasonography was normal, and she was started on oral prednisone 40 mg daily in addition to her previous regimen. The left eye remained uninvolved.
On day 5, she noted some improvement in her symptoms after starting the oral steroids. Her visual acuity improved to 20/150, and she had developed a hyphema. The remainder of her examination was stable.
On day 7, however, she returned with worsening symptoms despite good compliance with her treatment regimen. On examination, she was found to have decreased visual acuity to 20/200, no relative afferent pupillary defect, swelling and erythema of periorbital tissues, limited eye movements in all directions of gaze, and 3 mm of relative proptosis by Hertel exophthalmometry (Figure 1). Slit-lamp examination revealed edema of the upper and lower eyelids, conjunctival chemosis and hyperemia, marked injection of the scleral vessels without blanching, corneal edema with Descemet’s folds, hypopyon, persistent fibrinous anterior chamber reaction (4+ cell/1+ flare), and posterior synechiae (Figure 2). Examination of the left eye was unremarkable.
Posterior segment examination was notable for a hazy view but was otherwise normal. B-scan ultrasonography revealed thickened sclera and a T sign suggestive of posterior scleritis. Orbital computed tomography revealed circumferential scleral thickening of the right globe, proptosis, and minimal orbital fat stranding (Figure 3). There was no abscess formation or adjacent sinus disease. Laboratory workup for infectious or autoimmune etiologies revealed C-reactive protein of 12.3 mg/dL. The remainder of the laboratory results were unremarkable and included complete metabolic panel, complete blood count, chest x-ray, antinuclear antibodies, erythrocyte sedimentation rate, Borrellia burgdorferi antibodies, rapid plasma reagin, angiotensin-converting enzyme level, antineutrophil cytoplasmic antibodies, human leukocyte antigen B27, and thyroid function tests.
A diagnosis of bisphosphonate-associated orbital inflammation was made, and she was treated with one dose of methylprednisolone 1 g intravenous; her dose of oral prednisone was increased to 60 mg daily. Two days later, her symptoms were markedly improved and continued to improve over the following 10 weeks while steroids were tapered. Her visual acuity returned to 20/20, with complete resolution of her symptoms. The timeline of the clinical course is summarized in Table 1. | |
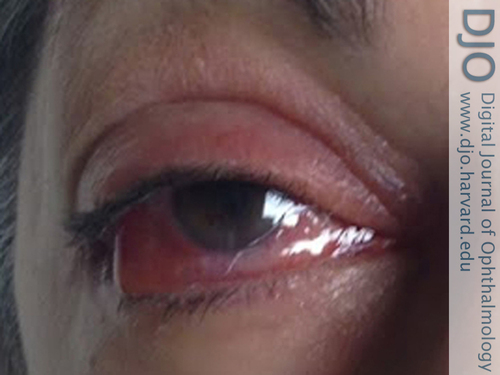
Figure 1
External appearance of the right eye 1 week after a presumed bisphosphonate-induced orbital inflammation notable for right-sided proptosis, ptosis, and conjunctival injection.
|
|
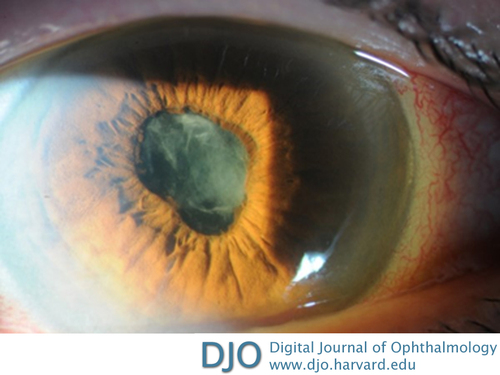
Figure 2
Slit-lamp photograph showing conjunctival injection, corneal edema with Descemet folds, fibrinous anterior chamber reaction in the pupil, and posterior synechiae with a pupillary membrane.
|
|

Figure 3
Orbital computed tomography showing right-sided scleral thickening (thin arrow), proptosis, and minimal orbital fat stranding (thick arrow) without extraocular muscle involvement.
|
|
| Discussion | To our knowledge, there have been 14 case reports of orbital inflammation after bisphosphonate administration. It has been reported secondary to alendronate (oral),(5) pamidronate (intravenous),(6,7) and zoledronate (intravenous).(8-18) It is often preceded by a prodromal illness consisting of fever, myalgias, and arthralgias; this acute-phase reaction has been reported in approximately 30% of patients who receive pamidronate and zoledronate infusions.(2,19) Orbital involvement after intravenous administration typically begins 2-6 days after infusion, and a concomitant, anterior uveitis has been seen in 29% of reports.(8-10,17,18,20) Recent reviews report some degree of bilateral involvement in 29% of cases and suggest this as a means of differentiating this entity form idiopathic orbital inflammation.8 In our patient, the temporal relationship of zoledronate infusion and symptom onset was much closer than previous reports of ocular and orbital inflammation following bisphosphonate therapy. Also, to our knowledge this is the first case of such severe and dramatic inflammation of the anterior segment and the orbit.
Bisphosphonates are inhibitors of osteoclastic bone resorption.(21) The proposed mechanism of ocular inflammation is an idiosyncratic γΔ T cell cytokine release involving IL-1 and IL-6 caused by bisphosphonates’ similar structure to pyrophosphate molecules.(22,23) This is based in-part on studies that show increased percentages of γΔ T cells in the peripheral blood of patients who experience an acute phase reaction after receiving pamidronate.(21)
In all cases of orbital inflammation, symptoms nearly completely resolve with intravenous steroids if begun in a timely manner, as seen with our patient.(8,20) Also of note, zoledronate rechallenge has been reported in a few cases, suggesting that tolerance may be seen with continued dosing with prophylactic steroids given to control initial symptoms.(8,11) However, given the severity of adverse reactions in the literature and in our patient, each case should be evaluated and the risks and benefits discussed with the patient. Also, high-dose steroids increase the risk of osteoporotic changes, and measures should be taken to reduce disease progression during steroid treatment, for example, prescribing the lowest effective steroid dose, encouraging weight-bearing exercises to prevent bone loss and muscle atrophy, and discouraging smoking and excess alcohol intake.
Although rare, orbital and ocular inflammation due to bisphosphonate therapy is a potential vision-threatening side effect. With the increasing use of bisphosphonates to treat osteoporosis in the aging population, as well as other disease entities, physicians must keep this complication in mind. The most common ocular involvement is typically mild and limited to nonspecific conjunctivitis, anterior uveitis, or blurred vision.(3) Patients with ocular and orbital inflammation should be questioned regarding recent bisphosphonate use. Patients may present with anterior segment inflammation including corneal endotheliitis and anterior uveitis as soon as 12 hours after treatment. Anterior segment inflammation may continue to progress despite oral steroids; intravenous steroids should be considered in such cases. Our case further strengthens a presumed but likely association between intravenous zoledronate and orbital and ocular inflammation. Greater awareness of this association may allow for earlier recognition and timely treatment of future cases.
Literature Search
The PubMed database was most recently searched in February 25, 2017, without language restriction, using the following search terms: bisphosphonate orbital inflammation, bisphosphonate uveitis, bisphosphonate scleritis, bisphosphonate panuveitis, zoledronate orbital inflammation, zoledronate uveitis, zoledronate scleritis, and zoledronate panuveitis. | | | References | 1. Lewiecki DEM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs 2011;71:791-814.
2. Fraunfelder FW, Fraunfelder FT, Jensvold B. Scleritis and other ocular side effects associated with pamidronate disodium. Am J Ophthalmol 2003;135:219-22.
3. Fraunfelder FW, Fraunfelder FT. Adverse ocular drug reactions recently identified by the National Registry of Drug-Induced Ocular Side Effects. Ophthalmology 2004;111:1275-9.
4. Fraunfelder F, Fraunfelder F. Drug-induced ocular side effects. 5th ed. Boston: Butterworth-Heinemann; 2001.
5. Mbekeani JN, Slamovits TL, Schwartz BH, Sauer HL. Ocular inflammation associated with alendronate therapy. Arch Ophthalmol 1999;117:837-8.
6. Ryan PJ, Sampath R. Idiopathic orbital inflammation following intravenous pamidronate. Rheumatology 2001;40:956-7.
7. Subramanian PS, Kerrison JB, Calvert PC, Miller NR. Orbital inflammatory disease after pamidronate treatment for metastatic prostate cancer. Arch Ophthalmol 2003;121:1335-6.
8. Peterson JD, Bedrossian EH. Bisphosphonate-associated orbital inflammation—a case report and review. Orbit 2012;31:119-23.
9. Phillips PM, Newman SA. Orbital inflammatory disease after intravenous infusion of zoledronate for treatment of metastatic renal cell carcinoma. Arch Ophthalmol 2008;126:137-9.
10. Sharma NS, Ooi J-L, Masselos K, Hooper MJ, Francis IC. Zoledronic acid infusion and orbital inflammatory disease. N Engl J Med 2008;359:1410-1.
11. Benderson D, Karakunnel J, Kathuria S, Badros A. Scleritis complicating zoledronic acid infusion. Clin Lymphoma Myeloma 2006;7:145-7.
12. Procianoy F, Procianoy E. Orbital inflammatory disease secondary to a single-dose administration of zoledronic acid for treatment of postmenopausal osteoporosis. Osteoporos Int 2010; 21:1057-8.
13. Seth A, Anderson DP, Albiani DA, Barton JJS. Orbital inflammation and optic neuropathy with zoledronic acid for metastatic prostate cancer. Can J Ophthalmol J Can Ophtalmol 2009;44:467-8.
14. Yang EB, Birkholz ES, Lee AG. Another case of bisphosphonate-induced orbital inflammation. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 2010; 30:94-5.
15. Missotten G, Verheezen Y. Orbital inflammation after use of zoledronic acid for metastasized prostate carcinoma. Bull Société Belge Ophtalmol 2010:23-4.
16. Yeo J, Jafer AK. Zolendronate associated inflammatory orbital disease. N Z Med J 2010;123:50-2.
17. Vora M, Rodgers R, Uretsky S. Nitrogen bisphosphonate-induced orbital inflammatory disease: gamma delta t cells—a report and review of 2 cases. Ophthal Plast Reconstr Surg 2014; 30:e84-5.
18. Pirbhai A, Rajak SN, Goold LA, et al. Bisphosphonate-induced orbital inflammation: a case series and review. Orbit 2015; 34:331-5.
19. Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc 2009;84:632-7; quiz 638.
20. Rahimy E, Law SK. Orbital inflammation after zoledronate infusion: an emerging complication. Can J Ophthalmol 2013; 48:e11-2.
21. Takeyama S, Shinoda H. Pharmacological actions and pharmacokinetics of bisphosphonates. Clin Calcium 2003;13:115-21.
22. McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013;126:13-20.
23. Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med 1999; 340:737-8. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in