|
|
 |
 |
 |
 |
|
|
Experimental use of an extracellular matrix graft in pterygium surgery
Digital Journal of Ophthalmology
2017
Volume 23, Number 4
December 28, 2017
|
Printer Friendly
Download PDF |
|
|


 Erik W. Anderson, MD
Erik W. Anderson, MD | Wake Forest Baptist Medical Center, Winston Salem, North Carolina Surendar Dwarakanathan, MD | John H. Stroger, Jr. Hospital of Cook County, Chicago Illinois Ramez Haddadin, MD | John H. Stroger, Jr. Hospital of Cook County, Chicago Illinois
|
|
|
| Abstract | | A 40-year-old man presented with a primary pterygium of the right eye and underwent pterygium excision using mitomycin C and placement of an extracellular matrix (ECM) adjuvant. As an adjuvant in pterygium surgery, ECM serves as a scaffold while promoting the growth of normal conjunctiva. Perioperatively, the ECM graft was found to be easily manipulated on the surgical field. It attached to the scleral bed with fibrin glue without complication. Postoperatively, there was no inflammation or local tissue reaction to the porcine ECM graft. At the most recent follow-up examination, 6 months postoperatively, there were no signs of recurrence of the pterygium past the limbus. This is the first report describing the use of ECM as an adjuvant to pterygium excision. | | | Introduction | | A pterygium is a fibrovascular growth originating from the conjunctiva extending onto the surface of the cornea. Clinically, pterygia cause irritation and redness and they induce astigmatism. Although excision of the focal area relieves symptoms, the primary challenge to successful surgical treatment of pterygium is recurrence. Several techniques have been introduced to reduce the incidence of recurrence, including use of mitomycin-C (MMC), conjunctival autograft, and amniotic membrane transplantation. An Ophthalmic Technology Assessment from the American Academy of Ophthalmology found a combination of two adjuvants: a limbal or conjunctival autograft with MMC resulted in a lower recurrence rate compared to the use of an autograft or MMC alone.(1) Despite these findings, they concluded that there is insufficient data to recommend a specific technique for each case. The purpose of this report is to introduce the use of extracellular matrix (ECM) as an adjuvant to pterygium excision. ECM is an acellular biomaterial that surrounds all cells, and it provides structural support. The collagens, proteoglycans, and glycoproteins of which it is composed play a critical role in the regulation of cellular differentiation and transformation.(2) The ECM used in this study is commercially available and made from porcine urinary bladder matrix. Animal- and human-derived ECM has been in use for over two decades to treat a wide range of conditions from reinforcing the abdominal wall in hernia repair to regrowing human muscle in traumatic war injuries.(3) In these cases, ECM serves as a scaffold while promoting growth of site-appropriate tissue. | | | Case Report | A 40-year-old man presented at Cook County Hospital with a primary pterygium of the right eye with irritation and redness (Figure 1A). The patient had undergone pterygium excision of the left eye 2 years prior at an outside hospital with subjective recurrence within 3 months. The patient’s best-corrected visual acuity was 20/30 in each eye. The pterygium on the right eye was thick and fleshy, with extension 5 mm past the limbus onto the cornea. The patient elected to undergo pterygium excision with ECM graft and preoperative subconjunctival injection of MMC. The operative procedure and use of extracellular matrix was reviewed by the Institutional Review Board of John H. Stroger, Jr. Hospital of Cook County. Given the on-label use, no IRB was required. ACell Matristem Wound Sheet (Matristem Wound Sheet, ACell Inc, Columbia, MD) received FDA 510K approval in October of 2009 for the management of surgical wounds, including graft sites. Recurrence was defined as any new fibrovascular growth across the limbus. The primary surgeon (EA) was a second-year ophthalmology resident at the time and was assisted by an experienced cornea fellowship–trained attending (RH).
One month before excision, MMC (0.1cc of 0.1mg/cc) was injected using a 30-gauge needle directly into the body of the pterygium in the subconjunctival space. No anterior segment photograph was taken after MMC injection; no clinical change in the pterygium prior to excision was observed by the authors on slit-lamp examination at 30 days after MMC injection, at which point the patient underwent pterygium excision. The head of the pterygium was bisected from the body of the pterygium along the limbus using Westcott scissors. The head of the pterygium was then grasped with 0.12 toothed forceps and dissected from the underlying cornea using a peeling technique. The cornea was polished with a crescent blade and diamond burr. Radial portions of the conjunctival wound margin were lifted with forceps and Tenon fascia was dissected to the origin of the medial rectus. An extensive Tenon fascia dissection past the medial rectus muscle to the orbital fat was not performed. The medical rectus muscle was not isolated. The 3 × 3.5 cm ECM graft was cut to a slightly larger size than the conjunctival defect and rehydrated in balanced salt solution for 10 minutes. The graft has an epithelial basement membrane on one surface and a lamina propria layer on the other. It was placed on the scleral bed with the basement membrane side up and attached using fibrin glue.
Postoperatively, the patient was started on topical prednisolone acetate 1% drops 4 times daily for 4 weeks, 3 times daily for 4 weeks, 2 times daily for 4 weeks, and finally once daily until follow-up at 6 months. Our regular taper schedule after pterygium surgery is 3 months. This patient’s taper was prolonged, given the quick recurrence after prior excision in the left eye, and the young age of the patient.
In the early postoperative period, there was no inflammation or local tissue reaction to the porcine ECM graft (Figure 1B-C). At 1 month there was remaining pterygium over the medical rectus muscle where dissection was not performed (Figure 1D). At 6 months, there were no signs of pterygium recurrence past the limbus, but superior blood vessels were extending onto the scleral bed (Figure 1E). Intraocular pressure was checked at every postoperative visit and showed no steroid response. | |
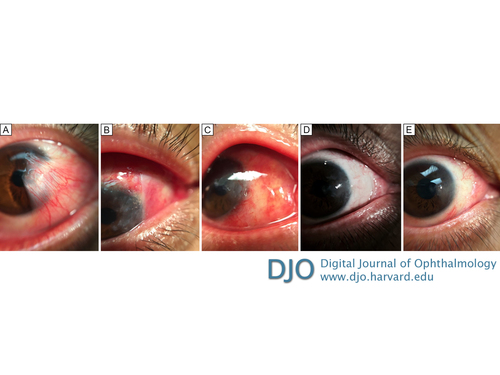
Figure 1
A, External photograph of the primary pterygium of the right eye. B, External photograph, postoperative week 1: the extracellular matrix (ECM) graft can be seen well attached to the scleral bed with opaque edges; the cornea and graft site is 50% epithelialized. C, External photograph, postoperative week 2: the entire graft is clear and attached to the scleral bed; the cornea and graft site are completely epithelialized. D, External photograph, postoperative month 1. E, External photograph, postoperative month 6.
|
|
| Discussion | Although the use of ECM as an adjuvant in pterygium surgery is a new technique, Meng et al have shown it to be an effective conjunctival substitute in an animal model.(4) In their study, a large conjunctival defect was induced in rabbits and repaired with an ECM graft. In serial examinations they found that at 1 week postoperatively blood vessels and a single layer of epithelial cells had migrated into the graft. At 4 weeks the graft surface was clear and covered by 2-3 layers of epithelial cells. At 8 weeks the ultrastructure of the regenerative conjunctival epithelium was indistinguishable from normal tissue based on electron microscopy examination. Also, a lymphocyte toxicity assay revealed no significant differences in the serum quantity of lymphocytes before and after transplantation of the graft, suggesting a low graft antigenicity.
The mechanism by which ECM induces new tissue growth and differentiation is complex and involves the interaction between a variety of growth factors and cytokines.(4) ECM comprises collagens that affect cell growth, proteoglycans that affect cell metabolism, and adhesive glycoproteins that influence cell migration and proliferation. In addition, the membrane acts as an inhibitory physical barrier that prevents the migration of fibrovascular tissue over the scleral bed.
Use of ECM in pterygium surgery offers several benefits. The dehydrated membrane is stored at room temperature and is relatively inexpensive ($84 for a 3 × 3 cm ECM sheet).(5) ECM is easy to handle in the surgical field. When the material is hydrated, it glides over tissue and is easily repositioned without introducing tears or folds in the tissue. In addition, using a bioengineered adjuvant rather than a conjunctival autograft results in a shorter surgical time and is more suitable for patients with a limited conjunctival reserve or those that may undergo a future glaucoma surgery.
In the present case, the patient, who had a history of pterygium recurrence in the contralateral eye, was kept on a prolonged course of a topical corticosteroid; nevertheless, as seen in Figure 1E, there was extension of superior blood vessels onto the scleral bed postoperatively. This extension was likely from pterygium tissue that remained after incomplete excision. Recently, Cornelius published a retrospective case series of 60 consecutive patients that underwent pterygium excision with extensive Tenon fascia dissection and found a low recurrence rate of 1.6%.(6) Accordingly, we have modified our surgical technique to include an extensive Tenon fascia dissection.
In conclusion, porcine ECM is a promising, cost-effective adjuvant to pterygium excision. ECM serves as a scaffold on which new conjunctival tissue growth can be constructed and facilitates anatomical and functional reconstruction to reduce pterygium recurrence. The application of ECM is new to ophthalmology, but the potential indications are numerous, including reducing adhesions in symblepharon repair,(7) healing of persistent corneal epithelial defects or perforations,(8) and remodeling of the corneal stroma in ectatic disorders of the cornea.(9)
Literature Search
The authors searched OVID and PubMed on May 1, 2017, for English-language results using the following terms: extracellular AND pterygium surgery. | | | References | 1. Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, Shtein RM. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology 2013;120:201-8.
2. Al-ubaidi M, Petroll M. Introduction to Special Issue on ocular ECM. Exp Eye Res 2015;133:1-2.
3. Fountain H. Human muscle, regrown on animal scaffolding. New York Times Research, Sept 16, 2012.
4. Meng X, Zhang Q, Tang S, Liu J. Experimental study and application of extracellular matrix of conjunctiva. Zhonghua Yan KeZaZhi 2001;37:207-10.
5. ACell Inc. Jessup, MD. Matristem Devices, http://acell.com/matristem-devices/.
6. Cornelius CR. Recurrence rate and complications of pterygium extended removal followed by extended conjunctival transplant. Cornea 2017;36:101-3.
7. Yang J. The Effect of cell-derived extracellular matrix membrane on wound adhesions in rabbit strabismus surgery. Tissue Eng Regen Med 2014;11:155-62.
8. Fujii A, Shearer TR, Azuma M. Galectin-3 enhances extracellular matrix associations and wound healing in monkey corneal epithelium. Exp Eye Res 2015;137:71-8.
9. Quantock AJ, Winkler M, Parfitt GJ, et al. From nano to macro: studying the hierarchical structure of the corneal extracellular matrix. Exp Eye Res 2015;133:81-99.
| |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in