|
|
 |
 |
 |
 |
|
|
Adenoid cystic carcinoma presenting with bilateral orbital extension without lacrimal gland involvement
Digital Journal of Ophthalmology
2018
Volume 24, Number 1
February 18, 2018
DOI: 10.5693/djo.02.2018.01.001
|
Printer Friendly
|
|
|


 Tayler M. Schwartz, MD
Tayler M. Schwartz, MD | Warren Alpert Medical School of Brown University, Providence Rhode Island Jeffrey M. Rogg, MD, FACR | Department of Radiology, Rhode Island Hospital, Providence, Rhode Island Rogers C. Griffith, MD | Department of Pathology, Rhode Island Hospital, Providence Rhode Island Michael E. Migliori, MD, FACS | Department of Ophthalmology, Rhode Island Hospital, Providence, Rhode Island
|
|
|
| Abstract | | Adenoid cystic carcinoma (ACC) is a rare neoplasm of secretory epithelium that most commonly occurs in the fifth and sixth decades of life. It is characterized by high recurrence rates and poor response to chemotherapy. In the orbit, ACC usually presents as a lacrimal gland mass. We describe the rare case of a 70-year-old woman who presented with pain during mastication and bilateral facial numbness in the cranial nerve V2 distribution. She was found to have adenoid cystic carcinoma involving the orbits bilaterally without lacrimal gland involvement and without a clear primary tumor. Imaging suggested that the tumor arose from the soft palate by extension along cranial nerves V2 and V3. The patient was treated with radiation therapy with some degree of radiographic improvement 27 months after diagnosis. This case emphasizes the importance of considering adenoid cystic carcinoma when evaluating orbital tumors sparing the lacrimal gland. We also suggest the possibility of an oropharyngeal source with anterograde intracranial extension in cases of putative primary orbital ACC without lacrimal gland involvement. | | | Introduction | | Adenoid cystic carcinoma (ACC) is a malignant neoplasm of secretory epithelium characterized by insidious local growth, high recurrence rates, and distant metastasis.(1) ACC most commonly occurs in the oral cavity, primarily in the minor salivary glands.(2) In the orbit, it almost always presents as a lacrimal gland mass.(3) We present a unique presentation of ACC with bilateral orbital involvement and sparing of the lacrimal glands. No primary tumor was found. We postulate that ACC arose from perineural extension along the skull base from the soft palate in this case. | | | Case Report | A 70-year-old woman with a remote history of renal cell carcinoma presented at Rhode Island Hospital with a 24-month history of progressive bilateral pain during mastication, bilateral facial numbness in the cranial nerve (CN) V2 distribution, and bilateral xerophthalmos. She also reported numbness and paresthesias in her feet bilaterally and chronic progressive weakness of the right lower extremity. Ophthalmic and neurologic examination revealed anisocoria, with the left pupil larger than the right, with no further abnormalities. Brain and orbits magnetic resonance imaging (MRI) with contrast revealed an enhancing lesion of the skull base extending to the orbital apex.
The patient was diagnosed initially with chronic leptomeningeal disease of unknown etiology and started on prednisone therapy. The differential diagnosis included central nervous system lymphoma, sarcoidosis, and metastatic leptomeningeal disease. Repeat brain and orbits MRI with contrast was initially read as normal and did not reveal orbital abnormalities. Further review revealed intracranial tumor extension with bilateral orbital involvement. As a result, the patient underwent biopsy of the right orbital lesion through a lateral orbitotomy 19 months after presentation.
The full extent of the intracranial and orbital involvement was clearer on retrospective review of the imaging after discussion at a multidisciplinary tumor board. Subsequent fat-suppressed, contrast-enhanced, T1-weighted axial MRI more clearly demonstrated bilateral posterior orbital enhancing tumors. Tumor extension was noted into bilateral pterygopalatine fossae and cavernous sinuses, with infiltration anteriorly along CN V2 within foramen rotundum. On the right, contiguous infiltration of the inferior orbital fissure to the orbit was identified. More inferiorly (at the level of the Vidian canal), there was enhancement of the superior aspect of the right pterygopalatine canal, consistent with tumor extension from the palate, the suspected source of the patient’s orbital ACC (Figures 1-2). Position emission tomography (PET) showed no evidence of malignancy in the brain or orbits. MRI of the spine also revealed a nonenhancing, T1- and T2-hypointense lesion at the L2 vertebral body, with a corresponding sclerotic lesion apparent on PET scan.
Pathology showed ACC with a mixed cribriform and tubular growth pattern infiltrating soft tissue, with no evidence of lacrimal gland or salivary gland tissue (Figure 3). The patient was determined not to be a surgical candidate and received 54 Gy of intensity-modulated radiation therapy to the retro-orbital areas and to the skull base. She was without clinical worsening and with some degree of radiographic improvement 27 months after diagnosis (Figure 4). The L2 vertebral body lesion remained stable after stereotactic radiosurgery. | |
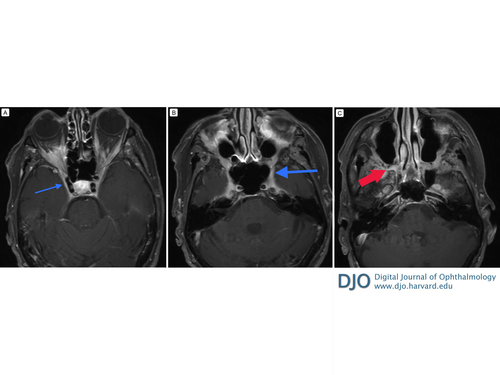
Figure 1
Axial, fat-suppressed, T1-weighted, axial magnetic resonance imaging (MRI) sequence with gadobutrol. A, Bilateral cavernous sinus enhancement (arrow) revealing the full extent of infraorbital involvement. B, At the level of the foramen rotundum (large arrow) there is enhancement extending from the cavernous sinus, foramen rotundum, pterygopalatine fossa, and inferior orbital fissure to the orbit bilaterally. C, At the level of the Vidian canal there is enhancement of the superior aspect of the pterygopalatine canal (red arrow), which represents a potential site of tumor extension from the palate.
|
|

Figure 2
Fat-suppressed, T1-weighted MRI sequence with gadobutrol. A, Coronal image showing enhancement of CN V2 in foramen rotundum bilaterally (arrows). B, Coronal image showing bilateral enhancement of the orbital apices and superior (large arrow) and inferior (small arrow) orbital fissures. C, Coronal image showing diffuse mass-like enhancement bilaterally, right (arrow) more than left, at the level of the retro-orbital fat. D, Sagittal image showing vertically oriented enhancing soft tissue (large arrow) extending from the palate to the pterygopalatine fossa within the pterygopalatine canal and tumor extending posteriorly into the cavernous sinus along the foramen rotundum and Vidian canal (medium arrow); the small arrow identifies the junction between the foramen rotundum and the inferior orbital fissure.
|
|
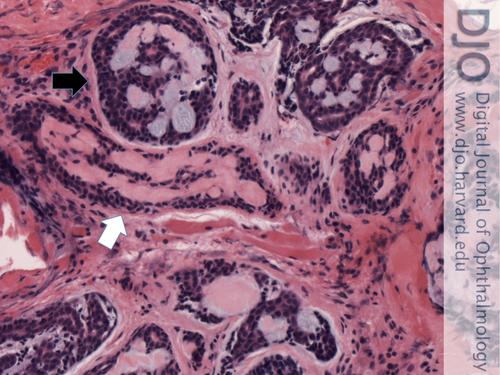
Figure 3
Infiltrating adenoid cystic carcinoma involving right orbital soft tissue. This malignant neoplasm has two growth patterns: cribriform (pseudocysts contain basophilic mucinous material in upper portion; black arrow) and tubular (tubules contain eosinophilic hyaline material in central portion; white arrow). Hematoxylin-eosin, original magnification ×200.
|
|

Figure 4
Most recent MRI, 10 months following treatment, showing improved but persistent thickening of the foramen rotundum and cavernous sinus and normalization of intraorbital masses.
|
|
| Discussion | ACC is a rare epithelial tumor, comprising 1% of all oral and maxillofacial tumors.(4) It occurs most commonly in the fifth and sixth decades of life(2) and can develop wherever there are secretory glands. The tumor can grow in cribriform, tubular, solid, or mixed patterns when differentiated. It is characterized histologically by angulated, hyperchromatic nuclei. ACC is also notable for its tendency toward hematogenous spread.(5) Surgery and postoperative radiation are the mainstay of therapy for ACC.(6) Intra-arterial chemotherapy has been used in head and neck cancers(7), and one center has used it to treat ACC of the lacrimal gland.(8) Further studies are needed to determine whether intra-arterial chemotherapy has a role in treating these potentially lethal cancers. ACC is known to recur at both local and distant sites years later, with one study showing a 53% recurrence rate a mean of 63 months after treatment.(9) Perineural invasion, increasing stage, and solid growth pattern have been found to be markers of poor prognosis.(10) One study found the mean 5-year survival rate to be 78.5%.(9)
ACC most commonly originates in the intraoral minor salivary glands,(2) and can rarely extend intracranially.(11) In the orbit, ACC usually arises from the lacrimal gland and is the most common malignant tumor of the lacrimal gland;(12) however, there was no evidence of lacrimal gland involvement in the present case. Although no primary tumor was evident, we mapped a possible course. Fat-saturated MRI of the orbits with contrast suggests that the tumor originated in the palate, with noted infiltration along CN V2 within the pterygopalatine canal to the pterygopalatine fossa, then into the foramen rotundum, where it likely spread through the inferior orbital fissures bilaterally to involve the posterior orbital soft tissues. Accordingly, the accessory salivary glands of the soft palate are the likely origin of the tumor.
Such a case of ACC with bilateral orbital involvement, without lacrimal gland involvement, and without a clear primary tumor is exceedingly rare. Our search of the literature, excluding reports where the lacrimal gland was grossly involved, found a single report of ACC extending to the orbits bilaterally;(13) Unlike the present case, there was a clear primary tumor in the paranasal sinuses evident on imaging.
Our case is also notable in that the tumor was difficult to detect intracranially. MRI with contrast, which did not include fat-suppression with orbital cuts, was initially read as normal. Additionally, PET scan of the brain and orbits did not show uptake, perhaps due to low metabolic activity of the tumor as supported by the patient’s lack of significant clinical worsening on long-term follow-up. ACC is known to pose significant diagnostic difficulty(14,15) due to its ability to be occult on imaging(16) and even histology.(15) It can appear isointense without contrast(17) and can spread with intervening areas of normal signal,(16,18) which may be partially explained by a proclivity toward early perineural invasion from the primary tumor site.(19) We believe that these characteristics of ACC, as demonstrated in the present case, may have implications for other reported cases involving the orbit.
There have been several cases reported of ACC that was thought to have originated primarily in the orbit, because there was no evidence of lacrimal gland involvement.(20-25) Zhang et al describe a 44-year-old man with ACC of the right orbit extending through the superior orbital fissure and a 34-year-old man with left orbital ACC with extension to the cavernous sinus.(21) Shields et al reported a 26-year-old man with ACC in the left nasal orbit with invasion of the medial rectus muscle and the tendon of the superior oblique muscle. On exenteration, the specimen had negative margins but contained an area of perineural invasion.(24) Walsh et al reported a 53-year-old woman with ACC of the left orbital apex extending into the left cavernous sinus.(23) Lin et al reported a a 60-year-old woman with ACC involving the inferior rectus muscle and extending to the inferomedial orbit.(20) Li et al reported a putative case of primary orbital ACC in a 70-year-old woman with a unilateral left orbital apex tumor with retrograde intracranial extension along the V2 nerve distribution, with no evidence of metastasis on PET scan.(22) Venkitaraman et al described a 51-year-old man who presented with orbital apex syndrome and a well-demarcated medial tumor in the left orbit displayed on MRI scan without enhancement on contrast; MRI scan 14 months later showed increased enhancement with contrast and displayed evidence of tumor involvement extending into the left cavernous sinus and Meckel’s cave.(25) Arsene et al suggested undetected lacrimal gland origin in a case of ACC involving the orbit in a 55-year-old woman who presented with a 0.66 cm left cavernous sinus tumor, with massive intracranial involvement involving the right cavernous sinus and orbit on computed tomography 3 months later. This patient was initially diagnosed with meningioma based on imaging and preliminary histological findings.(15)
In all of these cases, the authors postulate ectopic or occult lacrimal gland origin in these putative primary orbital cases of ACC.(15,20-25) We hypothesize that the tumors originated from an oropharyngeal source, with anterograde perineural intracranial extension to the orbit. As in the present case, the imaging findings may have been subtle and evaded detection. Walsh et al did consider the possibility of anterograde extension of the tumor from a sinonasal origin but considered this to be unlikely because no primary source was apparent after 13 months of follow-up.(23) We believe that the lack of a primary tumor in their case does not preclude sinonasal origin and note that repeat imaging in our patient did not reveal a primary tumor 19 months after diagnosis.
In conclusion, we report a case of ACC with bilateral orbital involvement without evidence of lacrimal gland origin and suggest, with no clear primary tumor, an oropharyngeal source based on imaging. This case not only highlights the importance of considering ACC in the differential diagnosis of orbital tumors without clear lacrimal gland involvement but also suggests a possible etiology in previously reported cases of seemingly primary orbital ACC without lacrimal gland involvement.
Literature Search
The authors search PubMed on June 1, 2016, for English-language results using the following terms: adenoid cystic carcinoma and orbit. | | | References | 1. Sung MW, Kim KH, Kim JW, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 2003;129:1193-7.
2. da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, de Almeida OP, Kowalski LP. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol 2006;42:139-46.
3. Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology 2004;111:997-1008.
4. Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck—a 20 years experience. Int J Oral Maxillofac Surg 2004;33:25-31.
5. Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med 2011;135:511-5.
6. Ahmad SM, Esmaeli B, Williams M, et al. American Joint Committee on Cancer classification predicts outcome of patients with lacrimal gland adenoid cystic carcinoma. Ophthalmology 2009;116:1210-5.
7. Homma A, Onimaru R, Matsuura K, Robbins KT, Fujii M. Intra-arterial chemoradiotherapy for head and neck cancer. Jpn J Clin Oncol 2016;46:4-12.
8. Tse DT, Kossler AL, Feuer WJ, Benedetto PW. Long-term outcomes of neoadjuvant intra-arterial cytoreductive chemotherapy for lacrimal gland adenoid cystic carcinoma. Ophthalmology 2013;120:1313-23.
9. Oplatek A, Ozer E, Agrawal A, Bapna S, Schuller DE. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope 2010;120:65-70.
10. Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. Int J Oral Maxillofac Surg 1997;26:435-9.
11. Alleyne CH, Bakay RA, Costigan D, Thomas B, Joseph GJ. Intracranial adenoid cystic carcinoma: case report and review of the literature. Surg Neurol 1996;45:265-71.
12. Weis E, Rootman J, Joly TJ, et al. Epithelial lacrimal gland tumors: pathologic classification and current understanding. Arch Ophthalmol 2009;127:1016-28.
13. Petrelli RL, Labay GR, Schwarz GS. Adenoid cystic carcinoma with orbital and cranial metastases: case report. Ann Ophthalmol 1978;10:611-5.
14. Seaver PR, Jr., Kuehn PG. Adenoid cystic carcinoma of the salivary glands. A study of ninety-three cases. Am J Surg 1979;137:449-55.
15. Arsene D, Ardeleanu C, D?n?il? L. Skull base tumor invading both cavernous sinuses. Adenoid cystic carcinoma mimicking a meningioma. Rom J Morphol Embryol 2006;47:367-71.
16. Ginsberg LE, DeMonte F. Palatal adenoid cystic carcinoma presenting as perineural spread to the cavernous sinus. Skull Base Surg 1998;8:39-43.
17. Dong J, Zhang L, Mo Y, Tian L, Liu L, Wu P. Discovery of invasion routes for nasopharyngeal adenoid cystic carcinoma. J Cancer 2015;6:90-7.
18. Lee YY, Castillo M, Nauert C. Intracranial perineural metastasis of adenoid cystic carcinoma of head and neck. J Comput Tomogr 1985;9:219-23.
19. Tse DT, Benedetto P, Morcos JJ, Johnson TE, Weed D, Dubovy S. An atypical presentation of adenoid cystic carcinoma of the lacrimal gland. Am J Ophthalmol 2006;141:187-9.
20. Lin SC, Kau HC, Yang CF, Yang MH, Tsai CC, Kao SC, et al. Adenoid cystic carcinoma arising in the inferior orbit without evidence of lacrimal gland involvement. Ophthal Plast Reconstr Surg 2008;24:74-6.
21. Zhang R, Qian J, Yuan Y, Bi Y. Atypical clinical presentation of orbital adenoid cystic carcinoma. J Cancer Res Ther 2015;11:1035.
22. Li B, Iordanous Y, Wang Y, Chakrabarti S, Allen LH. Adenoid cystic carcinoma presenting as an orbital apex mass with intracranial extension. Can J Ophthalmol 2016;51:e65-7.
23. Walsh RD, Vagefi MR, McClelland CM, Alonso-Basanta M, Newman JG, Farkas T, et al. Primary adenoid cystic carcinoma of the orbital apex. Ophthal Plast Reconstr Surg 2013;29:e33-5.
24. Shields JA, Shields CL, Eagle RC Jr, Adkins J, De Potter P. Adenoid cystic carcinoma developing in the nasal orbit. Am J Ophthalmol 1997;123:398-9.
25. Venkitaraman R, Madhavan J, Ramachandran K, Abraham E, Rajan B. Primary adenoid cystic carcinoma presenting as an orbital apex tumor. Neuroophthalmology 2008;32:27-32. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in