|
|
 |
 |
 |
 |
|
|
Pseudovitelliform maculopathy associated with deferoxamine toxicity: multimodal imaging and electrophysiology of a rare entity
Digital Journal of Ophthalmology
2017
Volume 23, Number 1
February 13, 2017
DOI: 10.5693/djo.02.2016.12.001
|
Printer Friendly
Download PDF |
|
|



Kelly Bui, MD | University of Southern California Eye Institute, Keck Medical Center of USC, Los Angeles, California SriniVas Sadda, MD | University of Southern California Eye Institute, Keck Medical Center of USC, Los Angeles, California; Doheny Eye Institute, University of California, Los Angeles, California Hani Salehi-Had, MD | Atlantis Eye Care, Huntington Beach, California
|
|
|
| Abstract | | Deferoxamine is a commonly used chelating agent for secondary hemochromatosis. We report a rare retinal manifestation of deferoxamine toxicity in a 68-year-old man and provide supporting multimodal imaging and electrophysiology. The patient had iron overload related to transfusion-dependent myelodysplastic syndrome and developed a pseudovitelliform macular lesion related to deferoxamine toxicity. We also describe for the first time the worsening of this maculopathy on deferasirox, an alternative chelating agent. Macular pseudovitelliform lesion is a unique manifestation of deferoxamine toxicity that can be mistaken for pattern dystrophy. It is important to recognize this manifestation, because discontinuation of the offending agent may halt or reverse the toxicity. | | | Case Report | A 68-year-old man presented to Atlantis Eyecare for evaluation of blurred vision in both eyes. He endorsed dark adaptation difficulties for 6 months. A year prior to presentation, he was noted by his general ophthalmologist to have a normal fundus examination. His past medical history was notable for myelodysplastic syndrome, for which he received frequent blood transfusions. He had been on deferoxamine for 5 years, presently at 16 g per week, for transfusion-related hemochromatosis. On review of systems, he reported adult-onset hearing loss. He had no family history of retinal degeneration or other pertinent history.
On examination, uncorrected Snellen visual acuity was 20/25-2 in the right eye and 20/30-2 in the left eye. Ishihara plates were 4/15 in the right eye and 1/15 in the left eye. There was no afferent pupillary defect. He had bilateral posterior chamber intraocular lenses. Fundus examination revealed healthy appearing optic nerves, but there was diffuse pigment mottling in both maculae, with pseudovitelliform lesions centrally (Figure 1A). Retinal vessels and periphery were unremarkable.
Fundus autofluorescence (FAF) revealed central hypo-autofluorescence, with surrounding punctuate increased and decreased autofluorescence in the macula (Figure 1B). Wide-field FAF did not reveal any abnormalities outside of the maculae (Figure 1C). Fluorescein angiography (FA) showed blocked fluorescence centrally, with surrounding punctate staining and transmission hyperfluorescence corresponding to the areas of pigment mottling (Figure 1D). Indocyanine green (ICG) angiography showed increased choroidal vascularity in both maculae (Figure 1E). Optical coherence tomography (OCT) of the macula revealed vitreomacular adhesion with central subretinal deposits in both eyes (Figure 2A).
Kinetic perimetry (Goldmann) revealed superior constriction in both eyes, worse on the right (Figure 3). Electroretinography (ERG) demonstrated a mild-to-moderate reduction in rod function, with relatively preserved cone function of the right eye and normal rod and cone function of the left eye (Figure 4A and 4B). The electro-oculogram (EOG) was markedly abnormal, with an Arden ratio of 1.3 in each eye (Figure 4C).
Given morphologic and functional evidence of retinal toxicity, deferoxamine was discontinued. Two months later, the patient’s vision was stable, and his symptoms had improved. It was noted that the pseudovitelliform lesions had decreased in size on OCT as evident both on the macular thickness map and by quantitative measurement of the central macular thickness (CMT; Figure 2B). The CMT had decreased from 380 to 337 in the right eye and from 385 to 336 in the left eye. Because of continued need for transfusions and the resulting elevated iron levels, the patient was started on deferasirox. The pseudovitelliform lesions increased in size on deferasirox (Figure 2C), with CMT increasing from 337 to 386 in the right eye and from 336 to 386 in the left eye. The visual acuity remained stable; however, the patient noted more difficulty with dark adaptation on the new treatment regiment. Deferasirox was discontinued, and he was started on brinzolamide in both eyes three times daily. At this point, the patient was lost to follow-up because of worsening of his systemic health conditions. | |
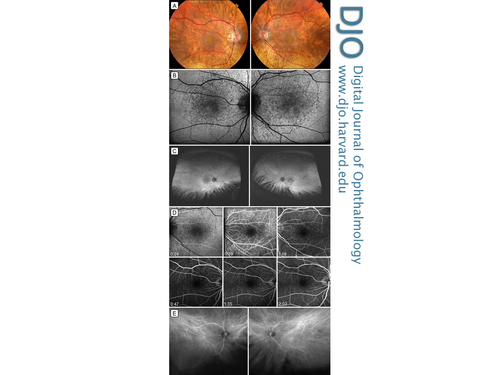
Figure 1
Multimodal imaging of deferoxamine-induced pseudovitelliform maculopathy. A, Fundus photographs of the right eye and left eye showing central macular pseudovitelliform lesions and surrounding pigment mottling. B, 30° fundus autofluorescence showing central, predominantly hypo-autofluorescence with surrounding areas of hyper- and hypo-autofluorescence corresponding to the pigment mottling. C, Wide-field autofluorescence demonstrating pigment mottling limited to the macula. D, Fluorescein angiography of both eyes showing blocked fluorescein centrally, and punctate staining and window defects corresponding to areas of pigment mottling. E, Indocyanine green angiography, mid-phase image, showing increased choroidal vascularity in both maculae (angiogram performed using the Daytona ultra-widefield scanner [www.optos.com] then being evaluated at the Doheny Eye Institute; exact timing of ICG not available).
|
|

Figure 2
Changes in pseudovitelliform lesions (white arrows) on optical coherence tomography. A, Optical coherence tomography showing pseudovitelliform lesions with both hypo- and hyper-reflective components on initial presentation while on deferoxamine. B, Optical coherence tomography showing a reduction in size of the pseudovitelliform lesions after discontinuation of deferoxamine. C, Optical coherence tomography showing an increase in size of the pseudovitelliform lesions with initiation of deferasirox.
|
|
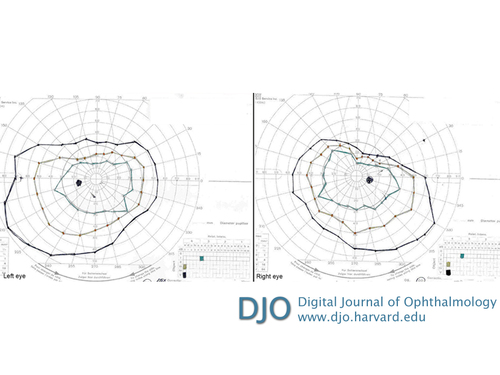
Figure 3
Kinetic perimetry (Goldmann visual field) showing superior field constriction in both eyes, but more severe in the right eye.
|
|
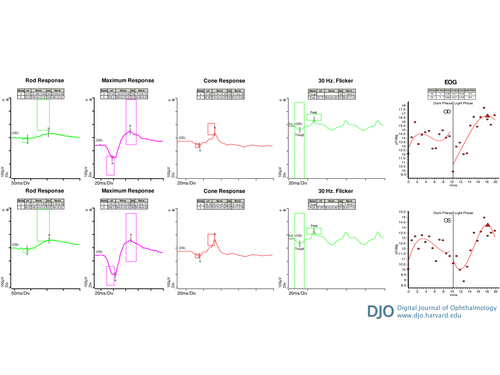
Figure 4
Electroretinogram showing a mild-to-moderate reduction in rod function of the right eye with relatively preserved cone function and normal rod and cone function of the left eye (a- and b- waves marked; boxes denote the normal limits for each peak). Electrooculogram showing markedly abnormal Arden ratio of 1.3 in each eye.
|
|
| Discussion | Deferoxamine is a commonly used iron-chelating agent that binds free iron in the blood stream and promotes its urinary excretion. Ototoxicity in the form of sensorineural hearing loss may occur, as seen in our patient. Various ocular toxicities have been reported, including cataract formation,(1) optic neuropathy,(2,3) and retinal damage, most commonly a retinal pigment epithelium (RPE) alteration,(3) which may resemble pattern dystrophy.(4) Pseudovitelliform lesions are rare, with only 4 reported cases to date.(4-6)
Deferoxamine toxicity has been associated with chelation of metals, particularly copper, which is essential to the normal function of the outer retina and RPE.(2,7,8) Histological evaluation8 has revealed enlarged RPE cells, some of which had retraction or complete loss of microvilli; some RPE cells showed ragged borders, loss of basal infolding of plasma membrane, and swollen mitochondria. The Bruch’s membrane underlying these degenerated RPE cells demonstrated abnormal thickening. In addition, deferoxamine may be directly toxic to the RPE cells.(9) The mechanism of development of the vitelliform lesion, however, is unknown.
Electrophysiologic testing frequently does not directly correlate with fundus findings. There are reports of earlier or more widespread injury on ERG/EOG than evident on fundus examination,(10) as well as cases(5,6,10) with normal electrophysiology despite pigment abnormalities or vitelliform lesions on examination.
Although not all case reports agree on a dose-related toxicity; it has been suggested that higher doses of deferoxamine over a prolonged period, along with a low serum ferritin level (eg, low iron store) can increase the risk of developing deferoxamine toxicity.(1-3) It is not known whether individual susceptibility factors and/or hereditary predisposition to pattern dystrophy may increase the risk of toxicity while patients are taking deferoxamine.
To our knowledge, this is the first report of pseudovitelliform maculopathy related to deferoxamine toxicity that is supported by complete multimodal imaging. Compared to previous reports,(4,5) our patient’s pseudovitelliform lesions were predominantly hypo-autofluorescent rather than hyper-autofluorescent. Previous reports described hyperfluorescence of the pseudovitelliform lesions on FA, ranging from staining to leakage,(5,6) contrasting with the hypofluorescence or blockage seen in our patient. This is also the first report of the ICG appearance of this entity. Deferoxamine toxicity leads to RPE damage, thickening of the adjacent Bruch’s membrane, and thus a likely change to the choriocapillaris. The choroidal hypervascularity seen on this patient’s ICG imaging likely indicates an ongoing inflammatory process. Although wide-field FAF in this case highlighted that RPE changes were limited to the maculae, ERG revealed diffuse rod dysfunction in the right eye. Similarly the EOG revealed diffuse dysfunction of the RPE in both eyes. Of the 4 previously described cases, 3 had ERG data and 2 had EOG data. Two cases described normal ERG,(5,6) and 1 described reduced cone function with intact rod function and a normal EOG.(6) We believe that the extent or degree of toxicity on the RPE and photoreceptor segments can explain the variable findings on ERG.
Currently there are three iron-chelating agents approved by the US Food and Drug Administration: deferoxamine (subcutaneous injection), deferasirox (oral), and deferiprone (oral). This is the first report of pseudovitelliform maculopathy that worsened on deferasirox therapy. The reason for this worsening is unknown; however, it may be advisable to consider deferiprone therapy for patients with pseudovitelliform maculopathy from deferoxamine toxicity.
It is generally accepted that regular screening for deferoxamine should be performed for patients on high doses or with prolonged exposure to deferoxamine; however, no generally accepted schedule has yet to be established. In addition to a dilated fundus examination, helpful ancillary tests include OCT, FA, FAF, ERG, EOG, color vision, and visual field testing. It has been suggested that a therapeutic index level can be calculated (safe level of <0.025) and may be a meaningful way to monitor for toxicity as it includes the daily dose per body weight (mg/kg) and takes into account the serum ferritin level (mg/L).(11) This is important because depletion of systemic iron stores may lead to chelation of other essential metals, such as copper, loss of which can subsequently lead to retinal toxicity. Discontinuation of the chelating agent generally leads to reversal of symptoms and signs; however, persistent damage has been reported,4,6 appearing to correlate with prolonged exposure. Genead and Fishman(12) reported a reduction in pseudovitelliform lesion using brinzolamide, although it is unclear whether the resolution was related to withdrawal of the offending agent or to brinzolamide.
Literature Search
PubMed was searched on August 21, 2015, for English-language articles (1975-present) using the following combinations of terms: retina, macula, retinal toxicity, retinopathy, maculopathy, vitelliform, or pseudovitelliform AND either deferoxamine OR deferasirox. | | | References | 1. Taneja R, Malik P, Sharma M, Agarwal MC. Multiple transfused thalassemia major: ocular manifestations in a hospital-based population. Indian J Ophthalmol 2010;58:125-30.
2. Olivieri N, Bunci JR, Chew E, et al. Visual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine infusions. N Engl J Med 1986;314:869-73.
3. Haimovici R, D’Amico DJ, Gragoudas ES, Sokol S; Deferoxamine Retinopathy Study Group. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology 2002;109:164-71.
4. Viola F, Barteselli G, Dell'arti L, et al. Multimodal imaging in deferoxamine retinopathy. Retina 2014;34:1428-38.
5. Genead MA, Fishman GA, Anastasakis A, Lindeman M. Macular vitelliform lesion in desferrioxamine-related retinopathy. Doc Ophthalmol 2010;121:161-6.
6. Gonzales CR, Lin AP, Engstrom RF, Kreiger AE. Bilateral vitelliform maculopathy and deferoxamine toxicity. Retina 2004;24:464-7.
7. Pall H, Blake DR, Winyard P, et al. Ocular toxicity of desferrioxamine—an example of copper promoted auto-oxidative damage? Br J Ophthalmol 1989;73:42-7.
8. Rahi AH, Hungerford JL, Ahmed AI. Ocular toxicity of desferrioxamine: light microscopic histochemical and ultrastructural findings. Br J Ophthalmol 1986;70:373-81.
9. Klettner A, Koinzer S, Waetzig V, Herdegen T, Roider J. Deferoxamine mesylate is toxic for retinal pigment epithelium cells in vitro, and its toxicity is mediated by p38. Cutan Ocul Toxicol 2010; 2:122-9.
10. Haimovici R, D'Amico DJ, Gragoudas ES, Sokol S. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology 2002;109:164-71.
11. Porter J B, Jaswon M S, Huehns E R, East CA, Hazell JW. Desferrioxamine ototoxicity: evaluation of risk factors in thalassaemic patients and guidelines for safe dosage. Br J Haematol 1989;73:403-9.
12. Genead MA, Fishman GA. Efficacy of brinzolamide ophthalmic suspension 1% for treatment of a vitelliform macular lesion in a patient with desferrioxamine retinopathy. Ophthalmic Surg Lasers Imaging 2011;42 Online:e114-7. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in