|
|
 |
 |
 |
 |
|
|
Surprises during intravitreal drug delivery—a report of three cases
Digital Journal of Ophthalmology
2017
Volume 23, Number 1
March 15, 2017
DOI: 10.5693/djo.02.2016.12.002
|
Printer Friendly
Download PDF |
|
|



Pradeep Venkatesh, MD | Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India Rohan Chawla, MD | Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India Bhavin Shah, MD | Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India Sat Pal Garg, MD | Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India
|
|
|
| Abstract | | We describe 3 cases with unusual events during and after intravitreal injection of dexamethasone intravitreal implants and bevacizumab. In case 1, delayed release of the safety stop of a dexamethasone intravitreal implant injector led to impaction and retinal break formation. Timely recognition and laser treatment prevented further retinal complications. In case 2, reinjection of a second dexamethasone intravitreal implant from the same site as the first injection was noted to dislodge the remnant of the previous implant from the vitreous base. In case 3, translucent floating particles were noted in the vitreous after intravitreal injection of bevacizumab from ampules procured from our dispensing pharmacy. | | | Introduction | | We describe 3 potentially harmful events seen after intravitreal injection of dexamethasone intravitreal implants and bevacizumab. All procedures were performed at the Dr. Rajendra Prasad Centre for Ophthalmic Sciences, AIIMS, New Delhi, from January 2011 to December 2011. To our knowledge, such events have not been reported previously. | | | Case Report | Case 1
A 60-year-old male diabetic patient with macular edema was scheduled to undergo intravitreal dexamethasone implant injection. During the procedure the surgeon forgot to pull out the safety stopper on the preloaded dexamethasone intravitreal implant injecting syringe. The surgeon was unable to release the implant. Realizing the mistake, the surgeon asked the assistant to remove the safety stop while the injecting needle was still in the eye. When the safety stop was removed, the implant was injected with great force, probably because the surgeon had applied unusual force on the injecting button. The implant went significantly posterior into the eye and impacted the retina, resulting in a thin layer of subretinal hemorrhage (Figure 1). After injection, the area of impact was laser delimited. At 24 months’ follow-up the patient was stable, with a small amount of subretinal fluid confined within the area of prophylactic laser demarcation. The macula, however, did show a reduction in the diabetic macular edema due to the effect of the steroid implant.
We recreated the above scenario on a model eye. The injector was introduced into the eye with the safety stop in place. In the first instance, we did not apply any pressure on the injecting button prior to removing the safety stopper. The safety stopper was removed with the needle in the model eye, and subsequently pressure was applied on the injecting button to release the implant. This led to the implant entering the eye as expected in the correct area. In the second instance, we applied continued pressure on the injecting button prior to removing the safety stopper with the needle in the eye. The safety stopper was removed by an assistant while the primary surgeon continued to apply pressure onto the injecting button. In the second scenario the implant penetrated deeper into the eye as it did in the patient (Figure 2).
Case 2
A 62 year old man with cystoid macular edema secondary to a branch retinal vein occlusion received an uneventful dexamethasone intravitreal implant 3 months earlier. The macular edema of the patient had reduced following the implant. However, because there was a recurrence of edema within 3 months, reinjection of a dexamethasone intravitreal implant was planned. The injection procedure of the second implant was performed in the same manner and approximately at the same site (4 mm inferotemporal to limbus) as the first implant. While performing the procedure with the aid of a microscope, the surgeon noticed a small, thinner implant floating posterior to the new one in the vitreous cavity (Figure 3). This seemed to be a result of dislodgement of the partially biodegraded matrix of the first implant, which had become relatively fixed in the peripheral vitreous base near the injection site; the pressure from the new implant was posited as the cause of its dislodgement. The concern of the operating surgeon was that a sudden movement of a foreign body from the vitreous base region could have caused traction and retinal dialysis/break formation. However, no retinal break or dialysis was noted at the injection site, and at 30 months’ follow-up, the patient had not developed any adverse retinal complication.
Case 3
A 55-year-old woman with a choroidal neovascular membrane underwent uneventful intravitreal injection of bevacizumab. At the 1-week follow-up, the visual acuity of the patient had improved, and there were no anterior chamber or vitreous cells. The intraocular pressure was normal. However, on dilated fundus examination, two small translucent particles were noted in the posterior vitreous (Figure 4). There was no sign of inflammation in the eye. On showing the images to our compounding pharmacy, we were informed that these could be inert carbon particles that migrated into the bevacizumab ampule during the sealing process. At 18 months’ follow-up, the particles remained in the eye without any evidence of inflammation. | |
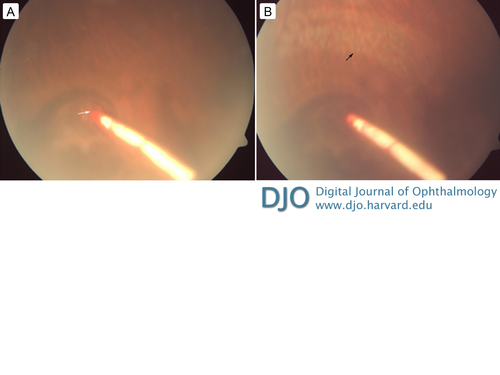
Figure 1
A, The impact of the dexamethasone intravitreal implant on the retinal surface (white arrow) with surrounding thin layer of subretinal hemorrhage. B, The impacted implant and the laser delimitation marks (black arrow).
|
|
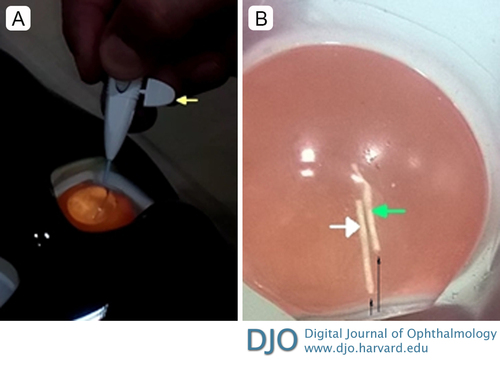
Figure 2
A, the procedure being performed on a model eye. The injector is shown inserted in the eye with the safety stopper in place (yellow arrow). B, The two implants inside the dummy eye. The implant marked by the white arrow was injected without applying pressure to the injector button at the time of removal of the safety stopper; the implant marked with the green arrow was injected while applying pressure to the injector button at the time of removal of the safety stopper. Thin black arrows show the path taken by the two implants. The implant marked by the green arrow is seen to penetrate deeper into the dummy eye.
|
|

Figure 3
The newly injected dexamethasone implant (white arrow) and the partially degraded matrix of the previous dexamethasone intravitreal implant (black arrow), which became displaced from the vitreous base due to the new implant.
|
|
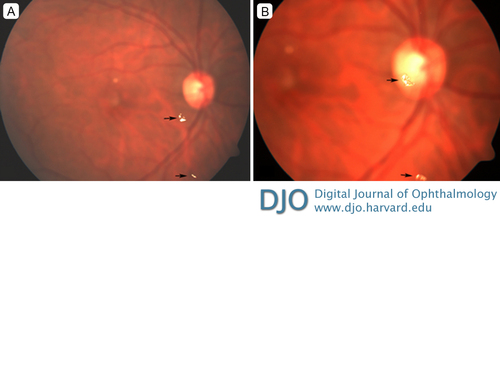
Figure 4
Two translucent particles (black arrows) floating in the vitreous (A); the media is clear with no evidence of intraocular inflammation. Magnified view of the same (B).
|
|
| Discussion | Discussion
Various complications following injection of dexamethasone intravitreal implants have been reported, including implant migration,(1) fractured dexamethasone intravitreal implant,(2) endophthalmitis,(3) and secondary glaucoma.(4) To our knowledge, impaction of the dexamethasone intravitreal implant and dislodgement of the remnant of a previously injected implant have not been reported.
In the first case, the surgeon forgot to remove the safety stop before entering the eye. While pressing the injection button, the safety stop was removed, resulting in the implant hitting the retina. Thus, care must be taken to remove the safety stop before injecting. If this situation does occur, it may be prudent to not apply any pressure on the injecting button and then remove the safety stopper to avoid a forceful injection of the implant into the eye. In case of the implant hitting the retina, the area of impact must be evaluated. In case a full thickness retinal break is suspected, the area of the break should be treated.
In the second case, the surgeon dislodged a fragment of the initial dexamethasone intravitreal implant from the vitreous base region at the time of the second implant injection. This could be potentially harmful, because the traction caused by a foreign body moving from the vitreous base could cause a retinal break. Hence, in a case where reinjection is required early, reinjection at the same site should be avoided. Also, the retinal periphery must be thoroughly examined to rule out any remnant of the previous implant at the intended site of reinjection.
In our third case, inert particles, possibly carbon in nature, were seen in the vitreous following intravitreal injection. Although these did not cause any inflammation or complications in our case, development of vitreous membranes and retinal detachment has been reported following intravitreal injection of carbon microparticles. These particles could enter the drug vials during processing at the compounding pharmacy. They are translucent and can be missed if few in number. Thus, it would be prudent to properly inspect the contents of vials packaged by dispensing pharmacies and the drug loaded in the syringe prior to injection.
Literature Search
PubMed was searched in English using the following key words: intravitreal injection, Ozurdex, dexamethasone implant, bevacizumab injection complications, intravitreal carbon particles, and complications following Ozurdex implant injection. | | | References | 1. Khurana RN, Appa SN, McCannel CA, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology2014;121:67-71.
2. Rishi P, Mathur G, Rishi E. Fractured OzurdexTM implant in the vitreous cavity. Indian J Ophthalmol2012;60:337-8.
3. Arıkan Yorgun M, Mutlu M, Toklu Y, Cakmak HB, Cağıl N. Suspected bacterial endophthalmitis following sustained-release dexamethasone intravitreal implant: a case report.Korean J Ophthalmol2014;28:275-7.
4. Kumari N, Parchand S, Kaushik S, Singh R. Intractable glaucoma necessitating dexamethasone implant (Ozurdex) removal and glaucoma surgery in a child with uveitis.BMJ Case Rep2013 Dec 5;2013. pii: bcr2013201293. doi: 10.1136/bcr-2013-201293.
| |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in