|
|
 |
 |
 |
 |
|
|
Value of anti-VEGF treatment in choroidal neovascularization associated with autosomal recessive bestrophinopathy
Digital Journal of Ophthalmology
2013
Volume 19, Number 4
December 30, 2013
DOI: 10.5693/djo.02.2013.09.001
|
Printer Friendly
Download PDF |
|
|


 Savitha Madhusudhan, FRCOphth
Savitha Madhusudhan, FRCOphth | St. Paul’s Eye Unit, Royal Liverpool University Hospital, Liverpool, United Kingdom Ahsen Hussain, FRCOphth | St. Paul’s Eye Unit, Royal Liverpool University Hospital, Liverpool, United Kingdom Jayashree Sahni, FRCOphth | St. Paul’s Eye Unit, Royal Liverpool University Hospital, Liverpool, United Kingdom
|
|
|
| Abstract | | A 26-year-old white woman presented with a 1-year history of reduced vision in both eyes, bilateral yellowish deposits in the central macula, and pale yellow retinal flecks extending to midretinal periphery. Choroidal neovascularization (CNV) was confirmed in her left eye. On optical coherence tomography, both eyes showed diffuse intraretinal cystic spaces, thickening and separation of the photoreceptor layer from the retinal pigment epithelium (RPE), subretinal fluid, and focal thickening at the level of the RPE at the fovea. A diagnosis of autosomal recessive bestrophinopathy was confirmed by electrodiagnostic and molecular genetics testing. The CNV responded well to intravitreal ranibizumab therapy, and visual acuity in the left eye improved and stabilized; however, retinoschisis due to fluctuations in intraretinal fluid persisted. This case highlights the fact that current optical coherence tomography–driven protocols used widely to treat neovascular age-related macular degeneration may not be appropriate for CNV associated with other retinal diseases. | | | Introduction | | Best dystrophy was formerly believed to follow an autosomal dominant inheritance pattern caused by mutation in the BEST1 gene. In 2008 Burgess et al described an autosomal recessive condition associated with biallelic mutations in the BEST1 gene, with either homozygous or compound heterozygous sequence variants, resulting in autosomal recessive bestrophinopathy (ARB).(1) In both Best dystrophy and ARB, yellow deep retinal/ retinal pigment epithelial deposits are present, and there is reduced light-rise on electrooculogram. ARB can also be associated with choroidal neovascularization (CNV) early in its course.(2) Little has been published on the treatment of vision-threatening manifestations of this condition. We report a case of ARB-associated CNV in a young patient, describe the spectral domain optical coherence tomographic imaging (Spectralis; Heidelberg Engineering, Germany) of the case, and show the outcome of treatment with intravitreal ranibizumab. | | | Case Report | A 26-year-old white woman presented to the eye primary care clinic at Royal Liverpool University Hospital with a 1-year history of worsening visual acuity in both eyes and recent onset distortion in her left eye. Her best-corrected visual acuity was 20/50 in the right eye and 20/80 in the left eye. Dilated fundus examination revealed bilateral yellowish deposits in the central macula. In addition, the left eye had a grayish elevated lesion at the fovea together with intraretinal hemorrhages (Figure 1). Multiple, autofluorescent, pale-yellow retinal flecks were seen extending to the midretinal periphery in both eyes (Figure 2). Fluorescein angiography showed petalloid hyperfluorescence at the fovea in the right eye suggestive of cystoid macular edema and characteristic early hyperfluorescence with late leakage in the left eye confirming the presence of a classic CNV (Figure 3). Spectral domain optical coherence tomography (SD-OCT) revealed diffuse intraretinal cystic spaces across both inner and outer plexiform layers, suggestive of intraretinal fluid, extending beyond the fovea; thickening and separation of the photoreceptor layer from the retinal pigment epithelium (RPE), subretinal fluid, and focal thickening at the level of the RPE in both foveae. Electrooculogram was reduced in both eyes, with an Arden ratio of 1.0; full-field electroretinogram (ERG) showed normal cone pathway responses but reduced rod responses. Multifocal ERG showed attenuated responses centrally in both eyes, the left being more affected than the right eye.
Given the above findings, a diagnosis of Best dystrophy complicated by CNV in the left eye was considered. Clinical examination and electrodiagnostic tests were normal in the patient’s parents. Molecular genetic testing was requested to confirm the diagnosis, with there being no family history of the above condition. Bidirectional fluorescent sequence analysis results revealed an autosomal recessive inheritance in the patient, with two pathogenic DNA variations: a frameshift mutation (c.878_883delinsC) in exon 8 and a sequence change (c422G>A (p. Arg141His)) on exon 4 of the BEST1 gene. This confirmed the diagnosis of ARB.
At our institution, treatment with 3 loading injections of intravitreal ranibizumab 0.5 mg/ 0.05 ml at monthly intervals is considered for all patients with choroidal neovascularization. This is followed by repeat injections, depending on the response. The classic CNV in the patient’s left eye was treated with 3 loading injections and did not require any additional treatment.
Visual acuity improved from 20/80 to 20/40 in the left eye and has been stable at 1 year’s follow-up. Fluorescein angiography at 3 months after commencing intravitreal treatment confirmed that the CNV was inactive, although dye leakage corresponding to the cystoid macular edema did not resolve. On SD-OCT, the subretinal fluid gradually decreased and cleared in the left eye. The intraretinal fluid (IRF) never resolved and after the first injection appeared to increase. During the 1-year follow-up period, the amount of IRF continued to fluctuate in both eyes. (Figure 4) Central foveal thickness at baseline and at 1 year was 476 ìm and 481 ìm in the right eye and 536 ìm and 531 ìm in the left eye. These changes did not seem to have a bearing on the visual acuity; her visual acuity in the untreated right eye remained stable at 20/50. | |
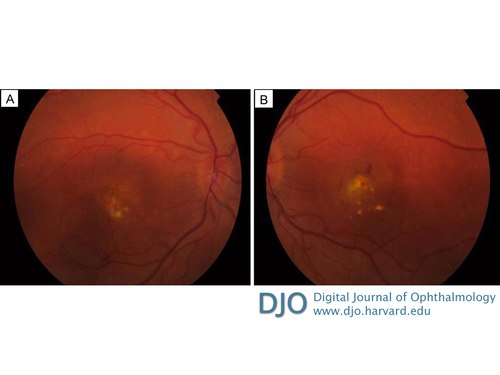
Figure 1
Color fundus photographs of right eye (A) and left eye (B) showing vitelliform deposits in both maculae and retinal hemorrhage in the left eye.
|
|
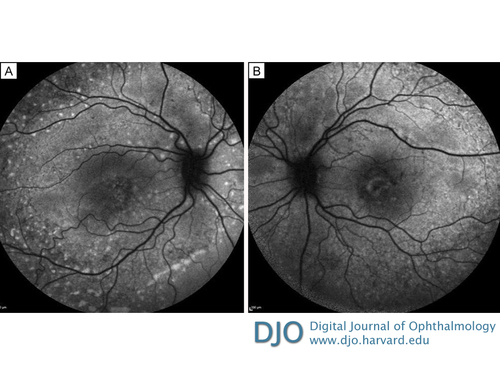
Figure 2
Fundus autofluorescence images of right eye (A) and left eye (B) showing multiple autofluorescent flecks extending to midperiphery.
|
|
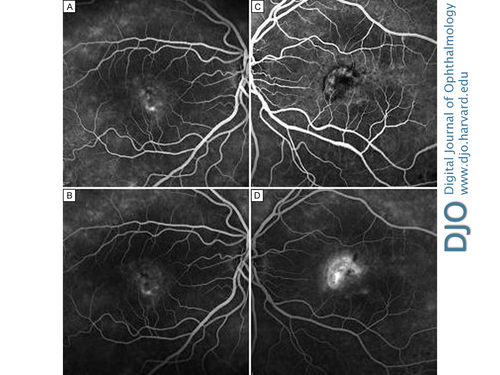
Figure 3
Fundus fluorescein angiogram of the right eye in the mid- (A) and late (B) venous phases at baseline, showing hyperfluorescence of vitelliform lesions and cystoid macular edema, and of the left eye in the mid- (C) and late (D) venous phases, showing active classic choroidal neovascularization.
|
|
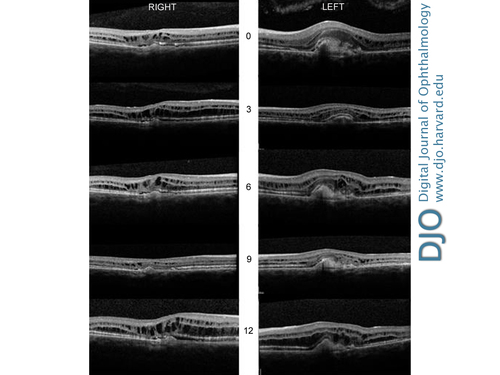
Figure 4
Serial spectral doman optical coherence tomography imaging at 0, 3, 6, 9, and 12 months in right and left eyes showing intraretinal hyporeflective spaces suggestive of fluid, thickened photoreceptor layer, and detachment of the retinal pigment epithelium.
|
|
| Discussion | ARB, or Burgess-Black retinopathy, typically presents early in life with central visual acuity loss. Clincially, there is diffuse RPE dysfunction beyond the macula, presence of subretinal and intraretinal fluid, atypical subfoveal vitelliform lesions together with extrafoveal and extramacular subretinal deposits.(1) BEST1 encodes bestrophin-1, a transmembrane protein primarily expressed in the basolateral membrane of the RPE. The functional role of bestrophin-1 within the RPE remains uncertain, with possible functions as a Ca2+ activated Cl− channel, a regulator of voltage gated Ca2+ channels, or as a HCO3− channel. It is postulated that before the photoreceptor outer segments are shed, large amounts of osmolytes are released, which adversely influence the role of bestrophin in RPE cell membranes leading to faulty phagocytosis and disrupted fluid transport across RPE.(3) This may explain the accumulation of lipofuscin and the development of cystoid macular edema. The rate of VEGF production from RPE cells is also thought to be dependent on the activity of voltage-dependent L-type Ca2+ channels and may be responsible for the early occurrence of CNV in ARB.(4)
Time-domain OCT findings in ARB are characterized by the presence of intraretinal cystic spaces and separation between the RPE and the neurosensory retina, possibly secondary to subretinal fluid accumulation, in addition to thickening of the RPE in areas corresponding to the vitelliform deposits.(1) SD-OCT in our patient showed the above changes and, thickening and detachment of the photoreceptor layer from the RPE. Using custom built, high-speed, high-resolution Fourier- domain OCT, Gerth et al have described elongated and thickened photoreceptor outer segments together with thin processes bridging the space between the photoreceptor and RPE layers in an 11-year-old boy with ARB.(5) Iannaccone et al found that bilateral intravitreal bevacizumab treatment in a 6-year-old child with ARB and associated CNV in one eye did not resolve the fluid fully, although the CNV responded favorably.(2) This is similar to our experience, where the classic CNV was no longer active on fluorescein angiography, but the intraretinal spaces persisted on SD-OCT. These schisis cavities in multiple retinal layers with bridging columns in both eyes resemble OCT changes seen in juvenile x-linked retinoschisis. The source of the intraretinal fluid is unknown and may not be directly related to the CNV. Therefore, current OCT-driven treatment protocols applicable to neovascular age-related macular degeneration,(6,7) advocating treatment until complete resolution of fluid is achieved may not be appropriate in CNV associated with causes other than AMD. This must be kept in mind when treating CNV associated with ARB. Successful treatment of visual loss in ARB requires a fuller understanding of the underlying pathogenesis; nevertheless, this case illustrates the utility of anti-VEGF therapy in treating CNV.
Acknowledgments
The authors thank the Ophthalmic Imaging staff at Clinical Eye Research Centre, St. Paul’s Eye Unit. | | | References | 1. Burgess R, Millar ID, Leroy BP et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet 2008; 82:19-31.
2. Iannaccone A, Kerr NC, Kinnick TR, Calzada JI, Stone EM. Autosomal recessive Best vitelliform macular dystrophy: report of a family and management of early-onset neovascular complications. Arch Ophthalmol 2011;129:211-7.
3. Xiao Q, Hartzell HC, Yu K. Bestrophins and Retinopathies. Pflugers Arch 2010; 460:559-69.
4. Rosenthal R, Heimann H, Agostini H, et al. Ca2+ channels in retinal pigment epithelial cells regulate vascular endothelial growth factor secretion rates in health and disease. Mol Vis 2007;27;13:443-56.
5. Gerth C, Zawadzki RJ, Werner JS, Héon E. Detailed analysis of retinal function and morphology in a patient with autosomal recessive bestrophinopathy (ARB). Doc Ophthalmol 2009;118:239-46.
6. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007;143:566-83.
7. Davis J, Olsen TW, Stewart M, Sternberg P Jr. How the comparison of age-related macular degeneration treatments trial results will impact clinical care. Am J Ophthalmol 2011; 152:509-14. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in