|
|
 |
 |
 |
 |
|
|
Keratoprosthesis in congenital hereditary endothelial dystrophy after multiple failed grafts
Digital Journal of Ophthalmology
2011
Volume 17, Number 3
September 23, 2011
DOI: 10.5693/djo.02.2011.06.002
|
Printer Friendly
Download PDF |
|
|


 Ramez Haddadin, MD
Ramez Haddadin, MD | Massachusetts Eye and Ear Infirmary and Department of Ophthalmology, Harvard Medical School Claes Dohlman, MD, PhD | Massachusetts Eye and Ear Infirmary and Department of Ophthalmology, Harvard Medical School
|
|
|
| Abstract | | Congenital hereditary endothelial dystrophy (CHED) has historically been managed with penetrating keratoplasty (PK), with moderate success, and, more recently, with Descemet’s stripping endothelial keratoplasty (DSEK). The possibility of repeated graft failures with CHED, however, makes alternative surgical procedures desirable. To our knowledge, this is the first reported use of a keratoprosthesis for management of CHED in a patient with multiple graft failures. The patient has been successfully followed for 5 years, has 20/30 vision, and no glaucoma. | | | Introduction | Congenital hereditary endothelial dystrophy (CHED) is characterized by early-onset, bilateral diffuse corneal edema and clouding due to dysfunctional corneal endothelium(1) Heredity has been described as either autosomal dominant or autosomal recessive. Corneal clouding may be present at birth, but in many instances the signs are delayed or mild. In severe cases, deep amblyopia may develop. Although CHED occurs worldwide, it seems to be more prevalent in the Middle East.(2)
The mainstay of management of advanced CHED cases is penetrating keratoplasty (PK).(3) The results have generally been described as "moderate"(2) but seem to have improved with time.(4,5) Decemet-stripping endothelial keratoplasty (DSEK) has also recently been attempted, with mixed results.(6,7) Under any circumstances, PK can fail repeatedly and, until recently, there has been no further treatment available. We present the first reported successful use of a keratoprosthesis (KPro) to treat CHED. | | | Case Report | An 18-year-old woman with CHED was referred to the Massachusetts Eye and Ear Infirmary after multiple failed corneal grafts in both eyes. She reported having her corneal problem since infancy, although her first PK was performed at 8 years of age. She since had repeated failed procedures, with 10 PKs in her right eye and 3 PKs in her left eye, never attaining functional vision between rehabilitation and reoperation. Two months prior to her visit, she experienced a vitreous hemorrhage in the left eye. Her brother also had CHED but with good vision after his initial PK as a child.
The patient’s vision was counting fingers in the right eye and hand motions in the left eye. Intraocular pressure (IOP) was 12-15 mm Hg by pneumotonometry in both eyes. Slit-lamp examination revealed a heavy corneal opacification in both eyes with stromal neovascularization. The right cornea was more significantly scarred. The pupil of the right eye did appear more distorted. Posterior chamber intraocular lenses were visualized. There was no view for funduscopic examination. B-scan ultrasound revealed an acoustically clear vitreous and an attached retina in both eyes.
The patient underwent placement of a Boston Keratoprosthesis type 1 (BKPro), pseudophakic, in the left eye in October 2005. The surgery was performed by one of the authors (CHD) according to previously described techniques.(8) The corneal graft was prepared with 8.5 and 3.0 mm trephines. The KPro front plate diameter was 6.0 mm and the stem diameter 3.35 mm. The back plate was 7.0 mm in diameter and 0.9 mm in thickness, with 8 holes, each 1.3 mm in diameter. The titanium locking ring had an outside diameter of 3.6 mm, inside diameter of 2.8 mm, and thickness of 0.32 mm. After trephination of the host with an 8 mm blade, the assembly was sutured in place with 16 10-0 nylon sutures. Peripheral iridectomy was performed prior to finishing suturing. A soft contact lens (Kontur, Kontur Kontact Lens Co, Inc, Hercules, CA), 16 mm in diameter, was placed on the eye. This contact lens arrangement, with periodic replacements, has been maintained without interruption (constant wear) ever since.
On postoperative day 1, the patient’s uncorrected visual acuity was 20/40. Her intraocular pressure was normal to finger palpation. She had little anterior chamber reaction. She was subsequently maintained on vancomycin eye drops (14 mg/ml), twice daily, moxifloxacin 0.5% twice daily, and prednisolone acetate 1% suspension four times daily. After several weeks, the patient developed redness, tearing, and mild ocular discomfort. The dosing frequency of antibiotics and corticosteroid was increased. At 5 weeks postoperatively, her vision had fallen to 20/200, and examination was otherwise notable for mild conjunctival injection and vitreous opacities. The presumptive diagnosis was sterile vitritis. Two peribulbar injections of 40 mg triamcinolone were administered with good response, although the IOP increased to 30-40 mm Hg as measured by finger palpation. Latanoprost, dorzolamide, and timolol were used temporarily. At 5 months postoperatively, the patient reported no discomfort nor any other problems. Her uncorrected visual acuity returned to 20/40. Examination revealed a quiet eye, with no evidence of vitritis. She continued on vancomycin, moxifloxacin, and prednisolone acetate twice daily with dorzolamide and timolol every night. IOP-lowering medications were subsequently stopped with maintenance of pressures in the normal range, again by finger palpation. Follow-up at 5 years revealed an uncorrected visual acuity of 20/30 and a quiet eye (Figure 1). The cup-to-disc ratio was 0.3. The patient was maintained on a daily dose of vancomycin and moxifloxin twice daily and prednisolone once daily. | |
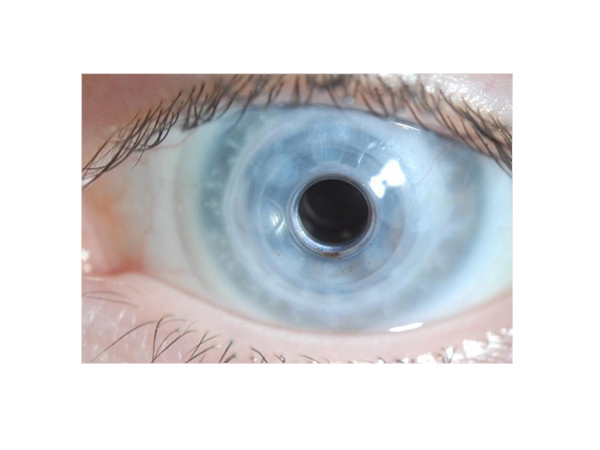
Figure 1
Postoperative photograph of the left eye 5 years after surgery with visual acuity of 20/30 (3 failed PKs had left the patient with a preoperative vision of hand movements). The intraocular pressure was normal.
|
|
| Discussion | For decades, management of CHED has been limited to PK. Several series have been presented with outcomes in terms of “survival” or “clear grafts,” and correlated with visual acuity over time. These definitions have not always turned out to be congruous, and a surprisingly large number of eyes with grossly clear grafts have been shown to have poor visual acuity. This disappointing outcome may be graft related, such as difficult-to-correct regular or irregular astigmatism, or possibly due to posterior complications or amblyopia. In general, PK in babies and small children is fraught with more complications than PKs performed at a later age.(5,9)
To put the KPro possibility in better perspective, we must turn to some recent reports of PK outcomes. Schaumberg et al. describe the results of PK in 16 eyes of 9 patients (6 eyes were operated on before one year of age).(4) A clear graft was reported in 11 of the 16 eyes; however, the “final” vision (median follow-up, 43.5 months) was found to be better than 20/400 in only 4 eyes. Failure was due to graft rejection in 2 eyes and to corneal ulcer in another 2 eyes. Four patients had preoperative nystagmus, which might indicate amblyopia. No retinal detachments were reported.
Al-Ghamdi et al described the results of PK in 35 eyes with CHED in Saudi Arabia.(5) On follow-up, 84% of the grafts had survived after 5 years; however, only half the number of eyes had a final visual acuity of 20/160 or better (4 eyes had a visual acuity of 20/40 or better). Still, the results in CHED were better than in some other congenital corneal opacities. Complications such as glaucoma, microbial keratitis, and vitreoretinal events were common.
DSEK in patients with CHED has also been reported.(6,7) DSEK directly addresses the pathophysiology of CHED and may eventually become part of the armamentarium for the management of this disease. At this point, the procedure remains technically difficult due to poor visualization as well as tightly adherent Descemet’s membrane, which complicates stripping.
We report only a single case and no meaningful comparison with PK can be made; however, the outcome of our case should give hope to CHED patients in whom PK has failed. The visual outcome of our patient has been excellent. Her course was only complicated by one episode of sterile vitritis, a reversible phenomenon of unclear etiology characterized by sudden, marked decrease in vision, with little or no pain, tenderness, conjunctival redness, or discharge, occurring in less than 4% of patients with the BKPro implanted.(10) We would not expect this phenomenon to be particularly more common in patients with CHED.
Even in the most successful surgical outcomes, both PK and DSEK require a considerable period of rehabilitation before optimal vision is achieved. Babies, who are at risk for deprivational amblyopia during this period, cannot wait for months to achieve a clear and stable cornea. Here a keratoprosthesis has a very distinct advantage over PK since the stable plastic allows more rapid attainment of final visual acuity.(11) Because of the amblyopia risk, several recent studies have encouraged the use of BKPro for patients with congenital corneal opacities in spite of technical difficulties in this age group.(12-14) This case report represents successful management of CHED in an adult after multiple failed grafts and outside the amblyopic period. Certainly in CHED, BKPro implantation deserves to be explored further, both in adult and pediatric patients.
Successful replacement of a failed graft with KPro in other forms of edema, usually in elderly people, has been documented many times. However, in CHED we feel that the situation is biologically very different. In general, the outcome of a repeat PK rarely depends on the state of the replaced failed tissue or on the quality of the new graft; rather, it is related to the state of the recipient. Thus the condition of the peripheral cornea (degree of edema and vascularization), the entire eye (degree of inflammatory response, immune privelege, etc), and the whole patient, including age (level of immune response, autoimmunity, etc), are the major determinants for the outcome of any regraft. The outcome of PK in CHED is still much inferior to that of PK in edematous corneas in elderly people, where the endothelial dysfunction is often restricted to the center of the cornea. In CHED, there is an absence of well functioning endothelium extending to the angle, and peripheral edema is greater as a result. In addition, general immune responses would be expected to be more enhanced in young CHED patients than in elderly ones. There may be other characteristics of CHED affecting treatment outcomes. Therefore we cannot assume that the KPro in our case should have the same favorable prognosis as in Fuch’s dystrophy. These relationships will have to be demonstrated clinically with a larger patient cohort with implanted KPros.
Acknowledgments
Financial support from the Massachusetts Eye and Ear Infirmary (MEEI) Keratoprosthesis Fund. | | | References | 1. Kenyon KR, Antine B. The pathogenesis of congenital hereditary endothelial dystrophy of the cornea. Am J Ophthalmol 1971;72:787-95.
2. Al-Rajhi AA, Wagoner MD. Penetrating keratoplasty in congenital hereditary endothelial dystrophy. Ophthalmology 1997;104:956-61.
3. Graham MA, Azar NF, Dana MR. Visual rehabilitation in children with congenital hereditary endothelial dystrophy. Int Ophthalmol Clin 2001;4:9-18.
4. Schaumberg D, Moyes AL, Gomes JAP, Dana RM. Corneal transplantation in young children with congenital hereditary endothelial dystrophy. Am J Ophthalmol 1999;127:373-8.
5. Al-Ghamdi A, Al-Rajhi A, Wagoner MD. Primary pediatric keratoplasty: indications, graft survival, and visual outcome. J AAPOS 2007;11:41-7.
6. Pineda II R, Jain V, Shome D, et al. Descemet’s stripping endothelial keratoplasty: is it an option for congenital hereditary endothelial dystrophy? Int Ophthalmol 2010;30:307-10.
7. Mittal V, Mittal R, Sangwan VS. Successful Descemet stripping endothelial keratoplasty in congenital hereditary endothelial dystrophy. Cornea 2011;30:354-6.
8. Dohlman CH, Nouri M. Keratoprosthesis surgery. In: Foster CS, Azar DT, Dohlman CH, eds. Smolin and Thoft’s The Cornea. Philadelphia, Lippincott Williams & Wilkins; 2005:1085-95.
9. Colby K. Changing times for pediatric keratoplasty. J AAPOS 2008;12:223-4.
10. Nouri M, Durand ML, Dohlman CH. Sudden reversible vitritis after keratoprosthesis: an immune phenomenon? Cornea 2005;24:915-9.
11. Dunlap K, Chak G, Aquavella JV, Myrowitz E, Akpek E. Short-term visual outcomes of Boston type 1 keratoprosthesis implantation. Ophthalmology 2010;117:687-92.
12. Botelho PJ, Congdon NG, Handa JT, Akpek EK. Keratoprosthesis in high-risk pediatric corneal transplantation: first 2 cases. Arch Ophthalmol 2006;124:1356-7.
13. Aquavella JV, Gearinger MD, Akpek EK, McCormick GJ. Pediatric keratoprosthesis. Ophthalmology 2007;114:989-94.
14. Nallasamy S, Colby K. Keratoprosthesis: procedure of choice for corneal opacities in children? Semin Ophthalmol 2010;25:244-8. | |
|
 |
 |
 |

|
|
 Welcome, please sign in
Welcome, please sign in  Welcome, please sign in
Welcome, please sign in