A 51-year-old woman with binocular diplopia and unilateral ptosis
Digital Journal of Ophthalmology 2019
Volume 25, Number 3
August 18, 2019
Volume 25, Number 3
August 18, 2019
Download PDF
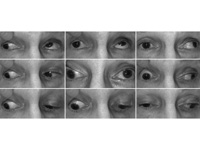
Figure 1.
Ocular motility testing demonstrating impaired adduction, supraduction, and infraduction of the right eye. Left eye motility was full.
Lumbar puncture for CSF analysis revealed a normal opening pressure (15 cm H2O) and elevated concentrations of glucose (103 mg/dL) and protein (58 mg/dL). White blood cell count was within normal limits and differential analysis revealed 25% monocytes and 47% blasts. Gram stain, culture, fungal studies, and viral studies were negative. Flow cytometry revealed 41% myeloid blasts, consistent with relapse of AML. The following day, bone marrow biopsy was performed, revealing no bone marrow involvement.
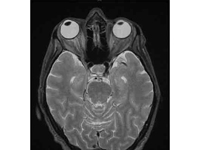
Figure 2
T2-weighted magnetic resonance imaging demonstrating mild right-sided proptosis and slightly increased cerebrospinal fluid signal surrounding the right optic nerve but no abnormal enhancement and no mass or retrobulbar inflammation.
T2-weighted magnetic resonance imaging demonstrating mild right-sided proptosis and slightly increased cerebrospinal fluid signal surrounding the right optic nerve but no abnormal enhancement and no mass or retrobulbar inflammation.
There was a brief reappearance of CSF myeloid blasts about 5 months after initiation of intrathecal chemotherapy that again resolved within 1 month. At 9 months, the patient’s bone marrow biopsy and CSF analysis revealed no phenotypic evidence of AML, and the patient was deemed in complete remission.
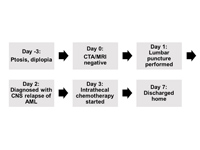
Figure 3
Timeline and hospital course. Imaging was obtained emergently to rule out aneurysm, followed by definitive diagnosis with lumbar puncture and treatment. AML, acute myeloid leukemia; CTA, computed tomography angiogram; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging.
Timeline and hospital course. Imaging was obtained emergently to rule out aneurysm, followed by definitive diagnosis with lumbar puncture and treatment. AML, acute myeloid leukemia; CTA, computed tomography angiogram; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging.
When aneurysm was ruled out via imaging in this patient with a history of malignancy and immunosuppression, the differential diagnosis was narrowed to metastatic or infectious processes. As illustrated in our case, these etiologies can be differentiated by lumbar puncture and CSF analysis.
Involvement of the bone marrow is usually seen in cases of AML CNS relapse, which occurs more often in those who have not undergone allogenic hematopoietic stem cell transplantation.(9) However, even in cases without bone marrow involvement and with prior stem cell transplantation, suspicion for leptomeningeal metastasis must be high in patients with focal neurologic deficits and a history of leukemia or lymphoma. MR imaging should be obtained but may be normal in 29%-88% of patients with leptomeningeal metastasis.(10-12) Thus, CSF sampling should be performed, because it is more sensitive, with a false negative rate as low as 11% (and lower with repeat sampling).(10,13) Current guidelines recommend CSF studies in conjunction with MRI in the initial workup of a patient with suspected leptomeningeal metastasis.(14-16) Once diagnosed, treatment consists of intrathecal chemotherapy with repeat clinical and CSF evaluation after induction therapy.(15)
To our knowledge, this is the first case of AML presenting as an isolated pupil-involving oculomotor nerve palsy due to leptomeningeal metastasis. Similar cases have been reported, but in those cases either the pupil was spared or other cranial nerves were involved in addition to the oculomotor nerve.(17-20) Furthermore, this is the only oculomotor nerve–involving case of AML relapse in which CNS involvement was not apparent on imaging. While similar manifestations of AML relapse have been described, all cases involving the oculomotor nerve presented with imaging findings correlating with clinically observed neurologic deficits.(17-19) Although our patient’s MRI revealed mild proptosis, signs of oculomotor nerve dysfunction as evident on examination were absent on imaging.
A similar case of abducens nerve–involving AML relapse not apparent on imaging was recently described by Fozza et al.(21) That case parallels the present one insofar as it was in a patient in their first AML remission presenting with CNS relapse manifesting as a cranial nerve palsy in the absence of imaging findings that was ultimately diagnosed with CSF evaluation. Our patient had undergone hematopoietic stem cell transplantation, though, whereas the patient reported by Fozza et al had not, making our case somewhat more atypical, because CNS relapse is less common after stem cell transplantation.(9) Moreover, their patient presented with bilateral abducens nerve deficits and systemic symptoms characteristic of intracranial hypertension, including nausea, vomiting, and dizziness. The abducens nerve is particularly susceptible to elevated intracranial pressure, and as such it is possible that generalized intracranial hypertension was the cause of the patient’s deficits rather than localized leptomeningeal invasion, as suspected in our patient, who only presented with an isolated, unilateral oculomotor nerve palsy and fatigue. Both patients illustrate the range of potential presentations and the importance of CSF evaluation in the assessment of a patient with suspected AML CNS relapse.
Literature Search
PubMed was searched on December 9, 2019, without date or language restriction, using the following terms, singly and in combination: acute myeloid leukemia AND leptomeningeal metastasis, oculomotor nerve, oculomotor nerve palsy, relapse, or third nerve palsy; oculomotor nerve OR oculomotor nerve palsy AND cancer, etiology, leukemia, leptomeningeal metastasis, and leptomeningeal lymphomatosis.
2. Jacobson DM. Relative pupil-sparing third nerve palsy: etiology and clinical variables predictive of a mass. Neurology 2001;56:797-8.
3. Sadagopan KA, Wasserman BN. Managing the patient with oculomotor nerve palsy: Curr Opin Ophthalmol 2013;24:438-47.
4. Alakel N, Stölzel F, Mohr B, et al. Symptomatic central nervous system involvement in adult patients with acute myeloid leukemia. Cancer Manag Res 2017;9:97-102.
5. Holmes R, Keating MJ, Cork A, et al. A unique pattern of central nervous system leukemia in acute myelomonocytic leukemia associated with inv(16)(p13q22). Blood 1985;65:1071-8.
6. Azzarelli V, Roessmann U. Pathogenesis of central nervous system infiltration in acute leukemia. Arch Pathol Lab Med 1977;101:203-5.
7. Cho WH, Choi YJ, Choi BK, Cha SH. Isolated recurrence of intracranial granulocytic sarcoma mimicking a falx meningioma in acute myeloblastic leukemia. J Korean Neurosurg Soc 2010;47:385-8.
8. Krendel DA, Albright RE, Graham DG. Infiltrative polyneuropathy due to acute monoblastic leukemia in hematologic remission. Neurology 1987;37:474-7.
9. Singhal S, Powles R, Treleaven J, et al. Central nervous system relapse after bone marrow transplantation for acute leukemia in first remission. Bone Marrow Transplant 1996;17:637-41.
10. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology 2010;74:1449-54.
11. Singh SK, Leeds NE, Ginsberg LE. MR Imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J Neuroradiol 2002;23:817-21.
12. Sze G, Soletsky S, Bronen R, Krol G. MR imaging of the cranial meninges with emphasis on contrast enhancement and meningeal carcinomatosis. AJNR Am J Neuroradiol 1989;10:965-75.
13. Nayar G, Ejikeme T, Chongsathidkiet P, et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget 2017;8:73312-28.
14. Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol 2017;19:484-92.
15. Chowdhary S, Chamberlain M. Leptomeningeal metastases: current concepts and management guidelines. J Natl Compr Canc Netw 2005;3:693-703.
16. Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin 2003;21:25-66.
17. Tabata M, Yoshida M, Takahashi H, et al. Oculomotor nerve invasion of acute myelogenous leukemia demonstrated by magnetic resonance imaging. Leuk Lymphoma 1998;30:411-14.
18. Haase R, Wiegand P, Hirsch W, et al. Unusual presentation of central nervous system relapse with oculomotor nerve palsy in a case of CD56-positive acute myeloid leukemia following allogeneic stem cell transplantation. Pediatr Transplant 2002;6:260-65.
19. Al-Mujaini AS, Al-Dhuhli HH, Dennison DJ. Acute unilateral third nerve palsy as an early manifestation of central nervous system relapse in a patient with acute myeloid leukemia. Saudi Med J 2009;30:961-3.
20. Saini JS, Mukherjee G, Mohan M. Acute myelomonocytic leukemia presenting as unilateral anterior cavernous sinus syndrome. Indian J Ophthalmol 1981;29:43-5.
21. Fozza C, Dore F, Isoni MA, et al. Strabismus and diplopia in a patient with acute myeloid leukemia. Am J Case Rep 2014;15:288-90.