A 71-year-old woman with decreased vision, nyctalopia, and peripheral vision loss
Digital Journal of Ophthalmology 2016
Volume 22, Number 4
December 31, 2016
DOI: 10.5693/djo.02.2016.06.001
Volume 22, Number 4
December 31, 2016
DOI: 10.5693/djo.02.2016.06.001
Download PDF
Dilated fundus examination of the right eye showed no vitreous cells, a titled, sharp, pink optic nerve, and a flat macula with a good foveal reflex. In the left eye, there were 2+ vitreous cells with a tilted optic nerve with temporal pallor and a trace epiretinal membrane in the macula. Both optic nerves had a cup-to-disc ratio of 0.1. Retinal vasculature in both eyes showed mild arteriolar attenuation.
Goldmann visual field testing of the right eye showed I-4e reduced to 20° circumferentially and V-4e with mild reduction superiorly to 50° and mild reduction temporally to 70° (Figure 1A); in the left eye, I-3e reduced to 10° circumferentially and III-4e and V-4e moderately reduced to 35°-40° superiorly and reduced inferiorly to 55°-60° (Figure 1B).
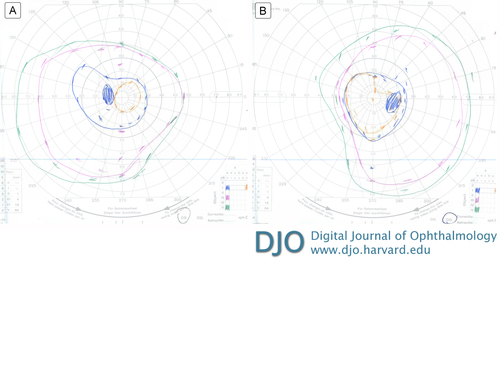
Figure 1
Goldmann visual field: left eye (A), right eye (B).
Goldmann visual field: left eye (A), right eye (B).
Full-field electroretinogram (ff ERG) in each eye showed, in photopic (light adaptation) conditions, normal a-wave and decreased b-wave (Figure 2A). The a-wave was −40.13 µV in the right eye and −40.67 µV in the left eye as well as 14 ms and 17 ms, respectively (normal range, −31.17 ± 20.34 µV and 12.87 ± 2.05 ms). The b-wave was abnormal, at 66.4 µV in the right eye and 72.48 µV in the left eye and 38 ms (normal range, 117.1 ± 43.44 µV and 29.81 ± 3.88 ms). In single-flash scotopic (dark adaptation) conditions, there were broadened a-waves and decreased b-waves as well as a decreased b/a ratio. (Figure 2B). The a-wave is −213.6 µV in the right eye and −224 µV in the left eye as well as 19 ms and 20 ms, respectively (normal range, −154.3 ± 73.08 µV and 15.5 ± 6.13 ms). The b-wave in contrast was abnormal. The b-wave measured to 133.7 µV in the right eye and 117.6 µV in the left eye and 105 ms and 97 ms, respectively (normal range, 286.6 ± 182.6 µV and 48.68 ± 8.52 ms).
Laboratory work-up included an evaluation for paraneoplastic disease. Serum sent to Athena Diagnostics (Marlborough, MA) were negative for the antiretinal autoantibodies against the 23kDa cancer-associated retinopathy (CAR) recoverin protein; but serum sent to the Ocular Immunology Laboratory at Casey Eye Institute (Portland, Oregon) were positive for the antiretinal autoantibodies against the 46kDa enolase protein.
Vitreous biopsy of the left eye showed atypical lymphocytes, inconclusive for vitreous lymphoma. The flow cytometry result demonstrated a “paucicellular specimen with scattered atypical lympocytes.”
Fluorescein angiogram showed no hallmark “leopard spot” changes at the level of the retinal pigment epithelium. Thus, the overall clinical picture was more suggestive of a smoldering low-grade vitritis rather than an ocular lymphoma.
Spectral domain optical coherence tomography (SD-OCT) of the maculae did not appear to show attenuation of the ellipsoid zone (Figure 3). Further fundus autofluorescence also did not show any ring maculopathy (Figure 4). Fundus photographs confirmed clinical examination findings, showing only a mild attenuation of the vessels with no apparent maculopathy in either eye (Figure 5).
A positron emission tomography computerized tomography (CT) scan showed hypermetabolic uptake in a 4.8 cm left axillary lymph node without any other pathologic uptake. A left axillary lymph node dissection revealed involvement of one lymph node for melanoma, whereas six additional lymph nodes were negative for metastatic disease.
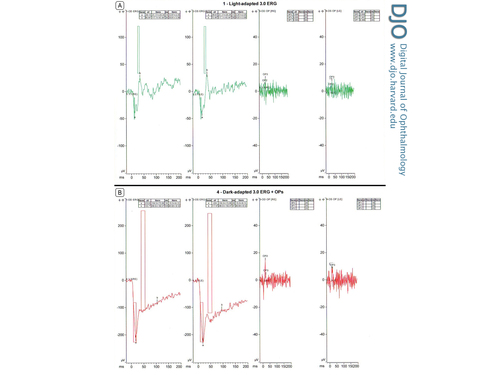
Figure 2
A, Photopic full-field electroretinogram (ffERG), right eye and left eye. B, Single-flash scotopic ffERG, right eye and left eye.
A, Photopic full-field electroretinogram (ffERG), right eye and left eye. B, Single-flash scotopic ffERG, right eye and left eye.
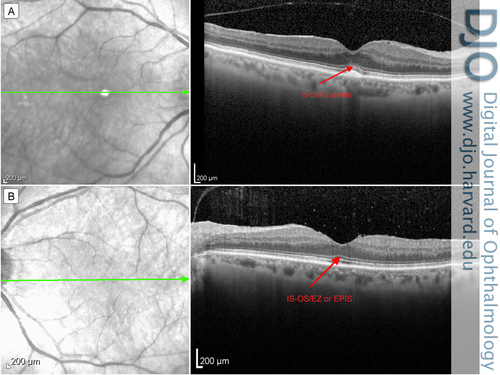
Figure 3
Optical coherence tomography of the macula: right eye (A) and left eye (B), with arrow indicating the inner segment–outer segment / ellipsoid zone or ellipsoid portion of inner segment (IS-OS/EZ or EPIS).
Optical coherence tomography of the macula: right eye (A) and left eye (B), with arrow indicating the inner segment–outer segment / ellipsoid zone or ellipsoid portion of inner segment (IS-OS/EZ or EPIS).
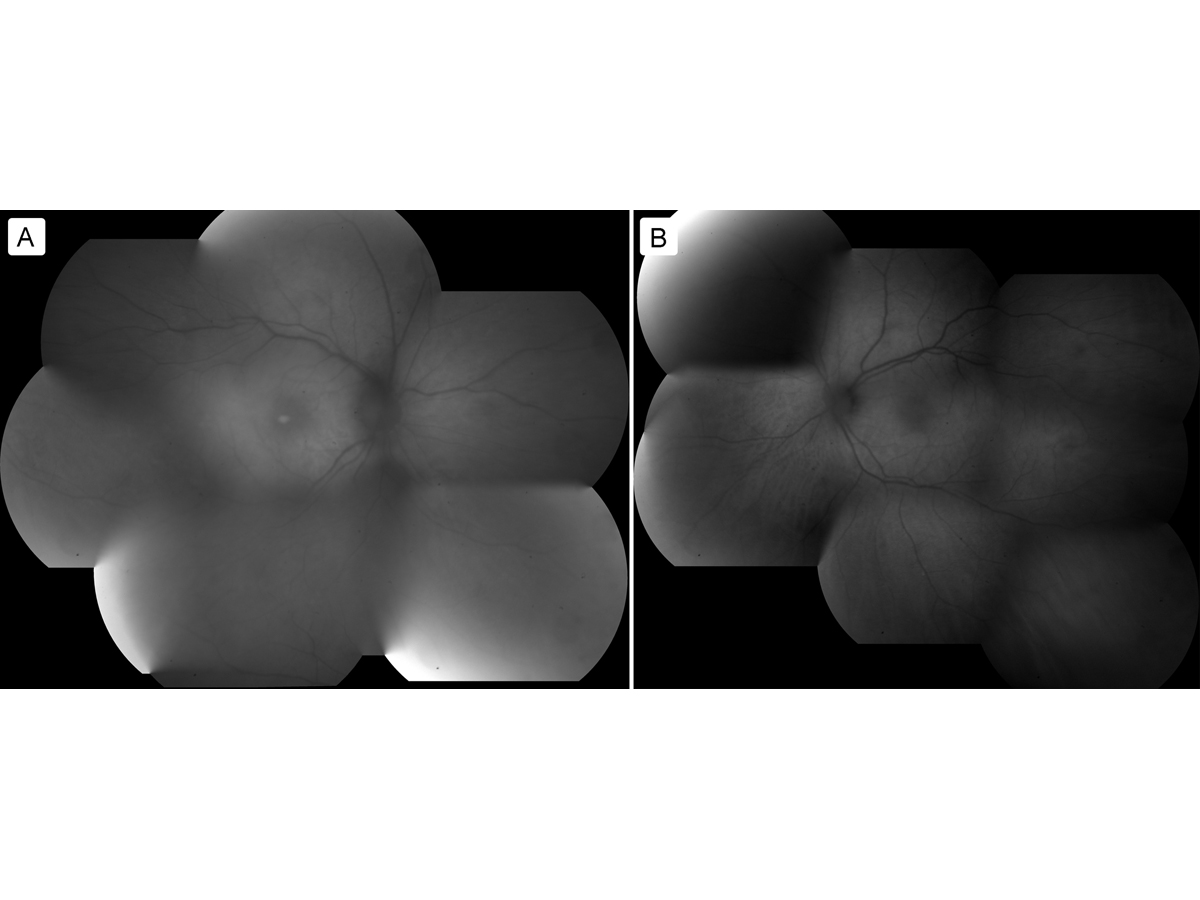
Figure 4
Fundus autofluorescence: right eye (A) and left eye (B).
Fundus autofluorescence: right eye (A) and left eye (B).
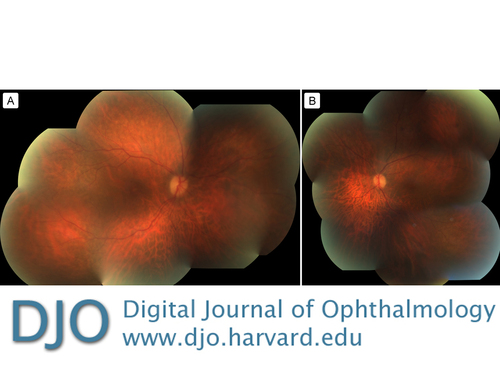
Figure 5
Fundus photographs of the right eye (A) and left eye (B).
Fundus photographs of the right eye (A) and left eye (B).
Our patient had positive antiretinal autoantibodies against the 46-kDa enolase protein, which is also associated with CAR; but, positive antiretinal autoantibodies are also found in 10% of healthy patients and in various autoimmune diseases.(5)
Our patient did not have any other paraneoplastic or antiretinal autoantibodies. The ffERG finding helped confirm the diagnosis of MAR. The proposed pathophysiology of MAR is due to antibodies which target the depolarizing bipolar cells.(6) In MAR, the photopic ffERG shows a decreased b-wave with a relatively normal a-wave, whereas the scotopic ffERG shows a markedly decreased b wave with decreased b/a wave ratio. In CAR, on the other hand, the photopic and scotopic ffERG shows a decreased a-wave and decreased b-wave.(7)
Both of these paraneoplastic syndromes can be treated with immunomodulation. Our patient was treated with intravenous immunoglobulin.(8) Although no large randomized trials exist, visual improvement in CAR has been reported in the majority of patients receiving corticosteroid treatment, although it has been noted that some patients with autoimmune retinopathies have improved with a combination of plasmapheresis and corticosteroid therapy.(1)
Visual loss in patients with a cancer history may be due paraneoplastic syndromes, treatment toxicity and ocular metastases. In addition to clinical evaluation, serologies and electrophysiology testing are important to establish a diagnosis. A diagnosis of a paraneoplastic disease should prompt evaluation of a patient for a primary cancer, metastatic disease, or progressing systemic cancer.
2. Goldstein SM, Syed NA, Milam AH, Maguire AM, Lawton TJ, Nichols CW. Cancer-associated retinopathy. Arch Ophthalmology 1999;117:1641-5.
3. Lu Y, Jia L, He S, et al. Melanoma-associated retinopathy: a paraneoplastic autoimmune complication. Arch Ophthalmology 2009;127:1572-80.
4. Myers DA, Bird BR, Ryan SM, et al. Unusual aspects of melanoma. Case 3. Melanoma-associated retinopathy presenting with night blindness. J Clinical Oncology 2004;22:746-8.
5. Grewal D, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: a review. Retina 2014;34:827-45.
6. Borkowski, LM, Grover S, Fishman GA, Jampol LM. Retinal findings in melanoma-associated retinopathy. Am J Ophthalmol 2001;132:273-5.
7. Lei B, Bush RA, Milam AH, Sieving PA. Human Melanoma Associated Retinopathy (MAR) antibodies alter retinal-ON response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci 2000;41:262-6.
8. Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin. Arch Ophthalmol 1999;127:612-4.