A 26-year-old man with a blind spot in his left eye
Digital Journal of Ophthalmology 2013
Volume 19, Number 3
September 26, 2013
DOI: 10.5693/djo.03.2013.07.001
Volume 19, Number 3
September 26, 2013
DOI: 10.5693/djo.03.2013.07.001
Download PDF
Dilated examination of the right eye was within normal limits. Examination of the left eye was pertinent for grade 1 vitreous haze (Figure 1) in the photographic scale described by Davis.(1) There was moderate optic nerve edema with adjacent flame hemorrhages (Figure 1). A small, 1/2 disc diameter area of nonspecific chorioretinal changes was noted in the inferotemporal midperiphery without associated retinochoroiditis.
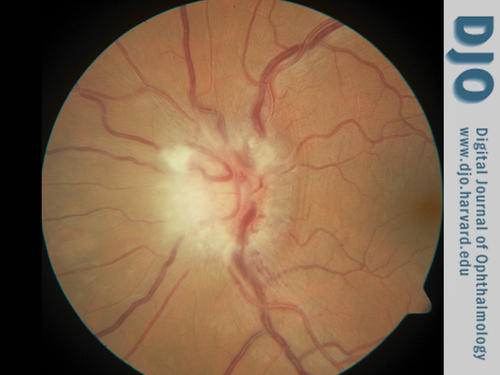
Figure 1
Color fundus photograph of the left eye. There is optic nerve edema and flame hemorrhage; vitreous haze, grade 1, was also appreciated.
Color fundus photograph of the left eye. There is optic nerve edema and flame hemorrhage; vitreous haze, grade 1, was also appreciated.
The workup of this patient included complete blood count, basic metabolic panel, B12, folate, angiotensin converting enzyme (ACE), antinuclear antibody (ANA), Bartonella and toxoplasma serologies, and fluorescent treponemal antibody (FTA-Abs). HIV testing was deferred. A lumbar puncture was performed, with an opening pressure of 20 cm H2O. Cerebrospinal fluid was analyzed for cryptococcal antigen, oligoclonal bands, Herpes simplex virus (HSV) 1 and 2 polymerase chain reaction (PCR), cytomegalovirus (CMV) PCR, and India ink.
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of the brain and orbits was performed with and without contrast, demonstrating normal signal of the optic nerves.
All of the test results were negative, except for Toxoplasma serologies, which were equivocal for Immunoglobulin M (IgM). This prompted repeat testing, which was positive for both IgG and IgM.
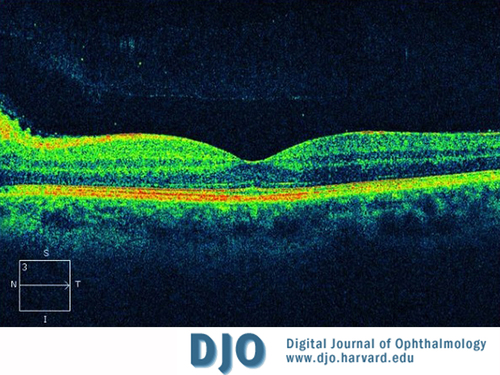
Figure 2
Macular optical coherence tomography (OCT) of the left eye showing peripapillary nerve fiber layer edema.
Macular optical coherence tomography (OCT) of the left eye showing peripapillary nerve fiber layer edema.
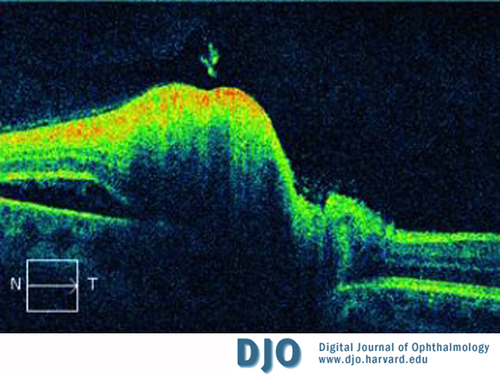
Figure 3
Peripapillary OCT of the left eye. Nerve fiber layer thickening and a neurosensory detachment nasal to the nerve are present.
Peripapillary OCT of the left eye. Nerve fiber layer thickening and a neurosensory detachment nasal to the nerve are present.
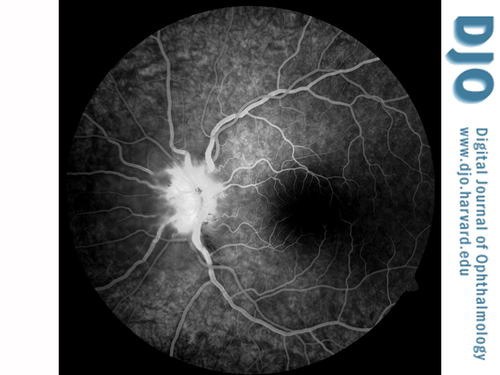
Figure 4
Fluorescein angiography of the left eye at 1:05.7 demonstrating hyperfluorescence and staining of the optic nerve.
Fluorescein angiography of the left eye at 1:05.7 demonstrating hyperfluorescence and staining of the optic nerve.
Fluorescein angiography findings typically include late phase disc staining. Capillaries supplying the optic nerve head demonstrate leakage that usually persists after resolution of the edema. Perifoveal capillary leakage is virtually nonexistent.(6)
Most cases of ocular toxoplasmosis are acquired instead of congenital.(7) The classic lesion in ocular toxoplasmosis is a focus of active retinochoroiditis adjacent to an old scar. The theory for the pathophysiology of this lesion is reactivation of Toxoplasma tissue cysts in the retina.(8)
Ultimately, our patient exhibited a toxoplasmic neuroretinitis, making this an atypical presentation of ocular toxoplasmosis. Other atypical forms include occlusive retinal vasculitis, retinal detachment, pigmentary retinopathies, panuveitis, optic neuropathy, and scleritis.(9) The presentations of toxoplasmosis are numerous and diverse.
Toxoplasma infection begins with ingestion of oocysts present in contaminated food and water sources.(4) While hematogenous spread is the most widely accepted route of infection, it has also been postulated that Toxoplasma can demonstrate a predominantly neuronal spread to the eye by way of the brain and optic nerve.(8) This is supported by the preponderance of juxtapapillary lesions seen in certain patient populations.(8) One retrospective study of 51 patients with active ocular toxoplasmosis with optic nerve involvement differentiated lesions into five categories:(10)
• Juxtapapillary retinochoroiditis—chorioretinal lesion contiguous with a swollen optic disc (33.3%)
• Pure papillitis—swollen optic disc, peripapillary vein sheathing plus a healed toxoplasmic scar (5.9%)
• Neuroretinitis—swollen disc, papillomacular or macular serous detachment, and hard exudates in the macula
• Distant lesion—swollen disc and a distant active lesion (43.1%)
• Mixed—any combination of the previous categories (15.7%)
The most serious potential complication of ocular toxoplasmosis is Toxoplasma encephalitis. This fatal complication is typically seen in immunocompromised hosts.11 A PubMed search revealed only one reported case of ocular toxoplasmosis with encephalitis in an immuncompetent person.(12) The patient had Toxoplasma retinochoroiditis without neuroretinitis. Despite the absence of large studies on the incidence of encephalitis in patients with Toxoplasma neuroretinitis, likely due to its rarity, one should have a high index of suspicion and rule-out brain involvement in all patients with neurologic findings. In fact, it may be prudent to obtain baseline neuroimaging in all patients with suspected Toxoplasma neuroretinitis.
Ocular toxoplasmosis remains a clinical diagnosis, typified by a focus of retinochoroiditis with vitreous inflammation and an adjacent chorioretinal scar. Serology, while useful in identifying primary infection, is limited in differentiating between recurrent acquired and recurrent congenital infection. IgM confirms primary infection, and remains positive for approximately a year. In contrast, IgG is detectable for life, in both congenital and acquired infections.(11) One study detected measurable rises of IgG in patients with active recurrent disease (typical and atypical) compared to patients with latent disease or seropositive nonspecific uveitis.(13) When serologies are equivocal, PCR analysis of intraocular fluid for parasites provides more robust diagnostic data.(14) Our patient was both IgG and IgM positive, which suggests a recently acquired infection.
Triple therapy for ocular toxoplasmosis is classically a combination of pyrimethamine, sulfadiazine, and prednisone. Due to high rates of intolerance and serious adverse effects, alternative treatment, such as trimethoprim/sulfamethoxazole and clindamycin, may be employed, and is supported by published trials yielding comparable results.(6,15)
Although toxoplasmosis rarely presents as neuroretinitis, the disease may have severe, even fatal, complications; thus it should be regularly considered as part of the differential diagnosis.
2. Cunningham ET Jr. What diseases should I consider in a patient with neuroretinitis? In Foster CS, Hinkle DM, Opremcak EM, eds. Curbside Consultation in Uveitis. Thorofare, NJ: Slack Book Incorporated; 2012:103-6.
3. Folk JC, Lobes LA. Presumed toxoplasmic papillitis. Ophthalmology 1984;91:64-7.
4. Gess A, Wender J, Jumper JM, Cunningham ET Jr. The red eye from Rio. EyeNet, May 2011:1-2.
5. Purvin V, Sundaram S, Kawasaki A. Neuroretinitis: review of the literature and new observations. J Neuroophthalmology, 2011;31:58-68.
6. Ray S, Gragoudas E. Neuroretinitis. Int Ophthalmol Clin 2001;41:83-102.
7. Holland GN. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol 1999;128:502-5.
8. Roberts F, McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol Today 1999;15:51-7.
9. Smith JR, Cunningham ET Jr. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol 2002;13:387-92.
10. Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye 2007;21:746-51.
11. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol 2005;20:129-41.
12. Waragai M, Takaya Y, Hayashi M. [A case of toxoplasmic chorioretinitis and meningoencephalitis in an immunocompetent adult]. Rinsho Shinkeigaku 1995;35:559-62.
13. Papadia, M., R. Aldigeri, and C.P. Herbort, The role of serology in active ocular toxoplasmosis. International Ophthalmology 2011;31:461-5.
14. Rothova A. Ocular manifestations of toxoplasmosis. Curr Opin Ophthalmol 2003;14:384-8.
15. Commodaro AG, Belfort RN, Rizzo LV, et al. Ocular toxoplasmosis—an update and review of the literature. Oswaldo CruzMem Inst 2009;104:345-50.