A 6-week-old baby boy with discharge
Digital Journal of Ophthalmology 2011
Volume 17, Number 3
August 15, 2011
DOI: 10.5693/djo.03.2011.07.001
Volume 17, Number 3
August 15, 2011
DOI: 10.5693/djo.03.2011.07.001
Download PDF
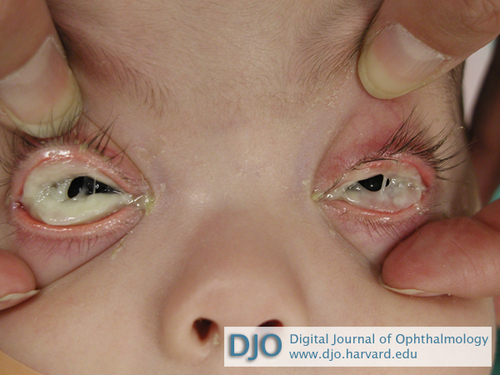
Figure 1
Thick, woody discharge overlying conjunctiva in both eyes.
Thick, woody discharge overlying conjunctiva in both eyes.
Figure 2
Amorpohous fibrinous material (broken arrow) and infiltrating inflammatory cells (straight arrow) in pseudomembrane tissue (hematoxylin-eosin, original magnification ×40).
Amorpohous fibrinous material (broken arrow) and infiltrating inflammatory cells (straight arrow) in pseudomembrane tissue (hematoxylin-eosin, original magnification ×40).
By 2 months after surgery, the heparin dosage remained unchanged and a small membrane had recurred along both lower lids. The new membrane was removed, and the patient subsequently started on Maxitrol (Neomycin sulfate 3.5mg, polymyxin B sulfate 10,000 units, dexamethasone 0.1%) and heparin 1000 U/ml, 1 drop each hourly in both eyes. The Maxitrol was tapered off by 2 months after surgery, and the heparin continued 1 drop every 3 hours; there was no recurrence at this time, but epiphora persisted and a mild entropion of the left lower eyelid was noted.
By 4 months after surgery the membrane had recurred along both lower eyelids and the left lower eyelid entropion persisted, as did the epiphora. A third membrane peel was performed. This time an amniotic membrane graft was placed along the palpebral conjunctiva of both lower lids. Postoperatively, prednisolone acetate, 1 drop hourly, moxifloxacin 4 times daily and heparin, 1 drop hourly, were started. The moxifloxacin was discontinued after 2 weeks and the prednisolone acetate tapered off over 2 months. At last follow-up, 7 months following the second membrane removal, there was no recurrence of membrane formation, the entropion and epiphora were improved, and the heparin (after a slow taper) was discontinued.
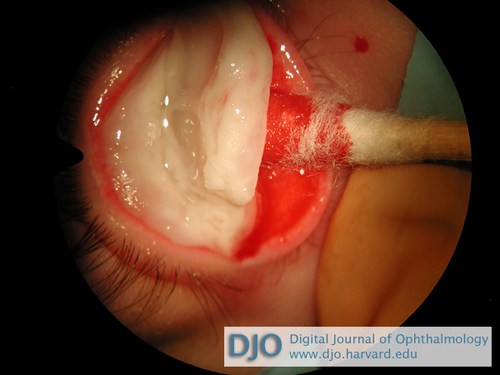
Figure 3
Removal of pseudomembrane.
Removal of pseudomembrane.
Pseudomembranes covering both eyes were first described by Bouisson in 1847. By 1933 the term ligneous conjunctivitis was proposed because the pseudomembrane had a woody appearance. Plasminogen deficiency was genetically linked with ligneous conjunctivitis in 1997.(1) A missense mutation at location K19E on the plasminogen gene (
The pathophysiology proposed for ligneous conjunctivitis is that a deficiency in plasminogen leads to deficient extravascular fibrin clearance, with wound-healing arrested at the granulation stage: minor insults to the conjunctiva may lead to an accumulation of the characteristic woody material.(4,5) The median age of clinical manifestation for plasminogen deficiency is 9.54 months, with ligneous conjunctivitis being the most frequently observed association, in 80% of cases, followed by ligneous gingivitis (34%), respiratory involvement (16%), and ligneous vaginitis (8%). Congential occlusive hydrocephalus has been associated with plasminogen deficiency in up to 8% of cases.(5) The clinical course of plasminogen deficiency depends on the site of involvement. When considering ligneous conjuncitivitis, erythema of the lid margin precedes epiphora, followed by development of woody conjunctival membranes. The condition is bilateral in roughly 50% of patients. Vision loss occurs in 20-30% of cases, primarily due to corneal involvement. Although most patients present as children, there are case reports of ligneous conjunctivitis in patients over age 55.
The natural history of these lesions is variable. Some resolve without treatment over months, whereas others may last many years. Patients with plasminogen deficiency may have a reduced life expectancy but this is most particular to the groups manifesting respiratory or cerebral involvement. Pseudomembranes involving the larynx or tracheobronchial tree can lead to recurrent pneumonia and airway obstruction. Pseudomembranes can also lead to a congenital occlusive hydrocephalus, which has a particularly poor outcome.(4,5)
Surgical excision often results in regrowth, as in our case, and many therapies have been attempted to prevent this, including amniotic membrane transplantation (AMT), local heparin, corticosteroids, cyclosporine, azathioprine, hyaluronidase, oral contraceptives, topical plasminogen, systemic plasminogen, fresh frozen plasma, and allogeneic serum drops. The AMT used in our patient prevented recurrence for 7 months. Barabino and Rolando first used AMT in the treatment of ligneous conjunctivitis; they reported no membrane recurrence 36 months postoperatively.(6) Recently, Lee and Himmel reported success combining allogeneic serum with topical heparin every two hours plus prednisolone acetate 1% drops 4 times daily.(1) Resolution of a ligneous membrane occurred without surgical intervention over a 2-month period, at which time all drops were discontinued. At 2 year’s follow-up there was no membrane recurrence. Many case reports describe good success with plasminogen drops; however this therapy is not available in the US.(1,2,4,5)
Acknowledgments
The author thanks Kyle Acosta, MD, Maria Vives, MD, and Mathew Stark, MD, for their assistance.
2. El-Darouti M, Zayed AA, El-Kamah GY, Mostafa MI. Ligneous conjunctivitis with oral mucous membrane involvement and decreased plasminogen level. Pediatr Dermatol 2009;26:448-51.
3. Mehta R, Shapiro AD. Plasminogen deficiency. Haemophilia 2008;14:1261-8.
4. Schuster V, Hügle B. Tefs K. Plasminogen deficiency. J Thromb Haemost 2007;5:2315-22.
5. Barabino S, Rolando M. Amniotic membrane transplantation in a case of ligneous conjunctivitis. Am J Ophthalmol 2004;37:752-3.