Three-dimensional telesurgery and remote proctoring over a 5G network
Digital Journal of Ophthalmology 2021
Volume 27, Number 3
July 26, 2021
Volume 27, Number 3
July 26, 2021
Download PDF
To present 2 cases of vitreoretinal surgery performed on a three-dimensional (3D) heads-up display surgical platform with real-time transfer of 3D video over a fifth-generation (5G) cellular network.
Methods
An epiretinal membrane peel and tractional retinal detachment repair performed at Massachusetts Eye and Ear in April 2019 were broadcast live to the Verizon 5G Lab in Cambridge, MA.
Results
Both surgeries were successful. The heads-up digital surgery platform, combined with a 5G network, allowed telesurgical transfer of high-quality 3D vitreoretinal surgery with minimal degradation. Average end-to-end latency was 250 ms, and average round-trip latency was 16 ms. Fine surgical details were observed remotely by a proctoring surgeon and trainee, with real-time communication via mobile phone.
Conclusions
This pilot study represents the first successful demonstration of vitreoretinal surgery transmitted over a 5G network. Telesurgery has the potential to enhance surgical education, provide intraoperative consultation and guidance from expert proctors, and improve patient outcomes, especially in remote and low-resource areas.
The recent COVID-19 pandemic has highlighted the benefits of virtualization of patient care and the effectiveness of virtual medical and surgical education.(7) Advances in digital surgical viewing technology and data transfer make telesurgery a promising tool. Three-dimensional (3D) digital surgical viewing systems allow surgeons to operate using a “heads-up” display monitor. Advantages include improved ergonomics for the surgeon, illumination, depth of field, and enhanced viewing experience for observing trainees and the surgical team.(8)
Recently, fifth-generation (5G) wireless cellular networks have become more widely available in various parts of the United States. 5G offers unprecedented network capability, in conjunction with a secure and reliable video streaming platform, allowing high-quality video streaming with reduced latency. Applications in telerobotic spinal surgery and telementoring in gastrointestinal surgery have demonstrated high satisfaction, efficacy, and feasibility of the 5G technology.(9,10) We present 2 cases of vitreoretinal surgery performed on a heads-up digital system with real-time transfer of 3D video over a 5G cellular network. Our experience shows that vitreoretinal digital 3D telesurgery may be broadcast over a 5G cellular network with fidelity.
Video was encoded and transmitted from the operating theater via wired public Internet to a Verizon 5G transmitter, wirelessly transmitted to a Verizon 5G receiver, then decoded and displayed at the Verizon Cambridge 5G Lab. Haivision commercial video encoding/decoding systems (CODECs) were used for low-latency video streaming, including the Makito X HEVC Encoder, Makito X HEVC Decoder and Secure Reliable Transport (SRT) Protocol. A CODEC compresses large video files into data packets for rapid transit via the Internet. Another CODEC expands the compressed packets to their original form, suitable for display on a remote monitor. SRT is an open-source protocol that allows for the delivery of high-quality, low-latency streams across the Internet (Figure 2). If a packet is lost, SRT is able to compensate by adding redundant information, increasing reliability at the cost of latency. SRT uses end-to-end 128/256-bit Advanced Encryption Standard (AES) encryption, which provides military grade security.
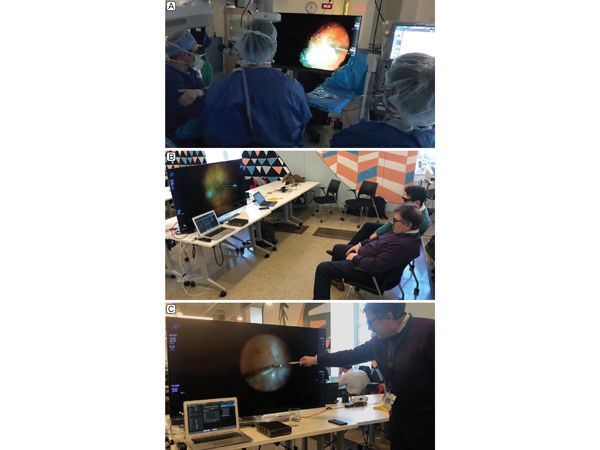
Figure 1.
Setup of telesurgery at primary and remote site. A, Operating team using the NGENUITY device at Mass. Eye and Ear. B, High-quality 3D video was live streamed to the proctoring surgeon and research fellow at the Verizon 5G Lab in Cambridge, Massachusetts. C, The proctoring surgeon pointed out fine details of the case observed in real time, including residual vitreous, a fine tractional membrane, and small retinal breaks.
Setup of telesurgery at primary and remote site. A, Operating team using the NGENUITY device at Mass. Eye and Ear. B, High-quality 3D video was live streamed to the proctoring surgeon and research fellow at the Verizon 5G Lab in Cambridge, Massachusetts. C, The proctoring surgeon pointed out fine details of the case observed in real time, including residual vitreous, a fine tractional membrane, and small retinal breaks.
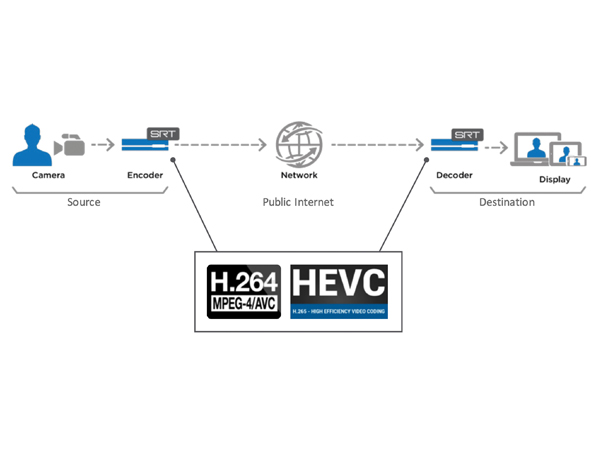
Figure 2.
Schematic of the video streaming protocol. Surgical video data is compressed by the encoder into data packets, which are transferred over a network and subsequently decompressed into its original form by the decoder and displayed at a remote site. SRT protocol provides secure, encrypted video streams and reliable transport of data by compensating for severe packet loss. H.264 and HEVC video encoders and decoders enable low latency end-to-end transport of secure, high quality video over unpredictable networks. Adapted, with permission, from Haivision (Deerfield, IL), “SRT Overview,” March 2020.
Schematic of the video streaming protocol. Surgical video data is compressed by the encoder into data packets, which are transferred over a network and subsequently decompressed into its original form by the decoder and displayed at a remote site. SRT protocol provides secure, encrypted video streams and reliable transport of data by compensating for severe packet loss. H.264 and HEVC video encoders and decoders enable low latency end-to-end transport of secure, high quality video over unpredictable networks. Adapted, with permission, from Haivision (Deerfield, IL), “SRT Overview,” March 2020.
In the first case, an epiretinal membrane (ERM) peel was performed. Triamcinolone was used to confirm the posterior vitreous detachment and remove the ERM with internal limiting membrane (ILM) forceps. Indocyanine green was used to stain the underlying ILM for removal to ensure adequate removal of the ERM across the central macula. To ensure that there were no peripheral retinal breaks, 360° scleral depression was performed. Both macular details and far peripheral details were well visualized by the proctoring surgeon at the remote viewing site in the Verizon 5G Lab in Cambridge, Massachusetts.
The second case involved tractional retinal detachment repair in a patient with proliferative diabetic retinopathy (Figure 3). The anterior-posterior vitreous connections were carefully removed for 360 degrees. Triamcinolone was injected to maximize vitreous removal. Subretinal fluid was drained through the superior retinal break and full panretinal photocoagulation was applied after fluid-air exchange. Silicone oil was placed for tamponade.
The proctoring surgeon and trainee easily observed fine details of the surgeries, including residual vitreous, a fine tractional membrane, and small retinal breaks. The operating surgeon and proctoring surgeon had a live discussion via mobile phone. In addition, the proctoring surgeon pointed out key features of the cases to the trainee using the remote monitor, such as careful segmentation and delamination of the posterior hyaloid. The NGENUITY displayed the instrument settings overlaid on the surgeon’s view, which was shared by the surgical team at Massachusetts Eye and Ear and the participants observing the case at the remote site.
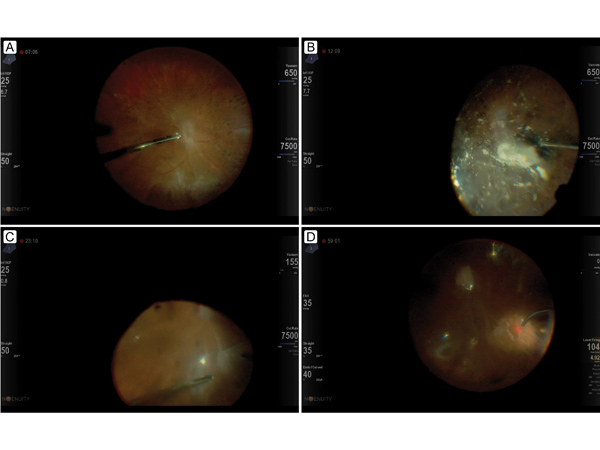
Figure 3.
Freeze frames from the tractional retinal detachment repair video showing the surgeon’s view, including the data fusion overlap of the surgical settings. A, Core vitrectomy, including removal of anterior posterior vitreous connections. B, Staining of the posterior hyaloid and residual vitreous with steroid. C, Segmentation of tractional membranes over the retinal detachment. D, Applying laser retinopexy around the superior retinal break and panretinal photocoagulation after fluid-air exchange.
Freeze frames from the tractional retinal detachment repair video showing the surgeon’s view, including the data fusion overlap of the surgical settings. A, Core vitrectomy, including removal of anterior posterior vitreous connections. B, Staining of the posterior hyaloid and residual vitreous with steroid. C, Segmentation of tractional membranes over the retinal detachment. D, Applying laser retinopexy around the superior retinal break and panretinal photocoagulation after fluid-air exchange.
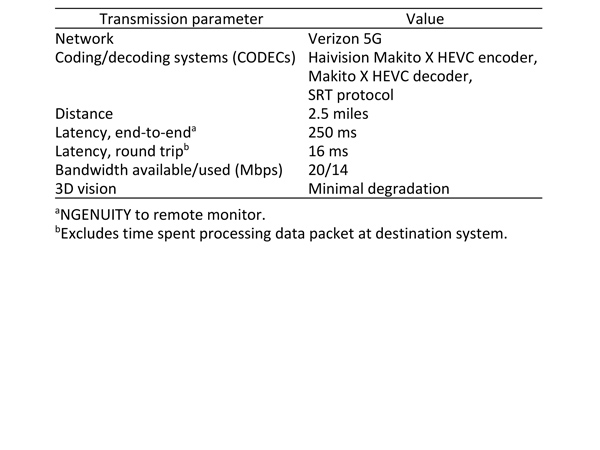
Table 1.
Results summary
Results summary
Remote real-time intraoperative high resolution video feeds can allow for on-call subspecialty surgeons to provide intraoperative guidance and recommendations for surgical cases, especially for more complex cases. Future adoption and integration of this technology may increase access to specialists and trainee education in low-resource areas. Undereducated or poor surgical performers may also benefit from intraoperative consultation from expert proctors under pay for performance and outcome-based payment models.(12) In addition, this model may be of utility in the military, where bases in remote areas lack access to specialty surgeons. Future work using higher bandwidths and complete 5G transmission from encoder to decoder without a wired internet connection is warranted.
Improvements in digital surgery platforms and data transmission will almost certainly pave the way for remote robotic surgery. While still in its infancy, robotic ocular surgery using the da Vinci robot has demonstrated feasibility in cataract surgery, strabismus surgery, and penetrating keratoplasty.(13-15) Robotic assistance has demonstrated improved dexterity, tremor cancelation and distance sensing, and these advancements will likely be applied to fine manipulations in vitreoretinal surgeries and later evolve into semiautonomous surgery.(16-19) Barriers to robotics in ophthalmic microsurgery include the fragility of eye anatomy, the high degree of precision required, and unexpected head movement by the patient during surgery.(20)
Limitations to telesurgery include the upfront cost of digital platforms, hesitancy to change surgical practice, and security concerns using public networks. While the current requirement for both a microscope and the 3D display has created a barrier to entry, this technology is expected to become more affordable over time when the 3D display becomes integrated with a single platform microscope. A single-center, randomized study reported longer macular peel times using a heads-up display surgical platform compared to a standard operating microscope, likely explained in part by the learning curve inherent in using new technology.(21) Notably, total operative time, complication rate and postoperative outcomes were comparable. We believe investments in telesurgery will ultimately improve the quality of care for patients, satisfaction among surgeons, and quality of education for trainees. We encourage providers to innovate with telesurgery as digital platforms and data transfer technology continue to improve.
Disclosures
Drs. Houston and Miller are consultants for Alcon, whose digital viewing system was used in these surgical cases. John B. Miller and S.K. Steven Houston III report personal fees from Alcon, outside the submitted work.
2. Lee CS, Morris A, Van Gelder RN, Lee AY. Evaluating access to eye care in the contiguous United States by calculated driving time in the United States Medicare population. Ophthalmology 2016;123:2456-61.
3. Zambelli-Weiner A, Crews JE, Friedman DS. Disparities in adult vision health in the United States. Am J Ophthalmol 2012;154(6 Suppl):S23-30.e1.
4. Desai N, Copeland RA. Socioeconomic disparities in cataract surgery. Curr Opin Ophthalmol 2013;24:74-8.
5. Nathan N, Joos KM. Glaucoma disparities in the Hispanic population. Semin Ophthalmol 2016;31:394-9.
6. Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The current state of teleophthalmology in the United States. Ophthalmology 2017;124:1729-34.
7. Saleem SM, Pasquale LR, Sidoti PA, Tsai JC. Virtual ophthalmology: telemedicine in a COVID-19 era. Am J Ophthalmol 2020;216:237-42.
8. Agranat JS, Miller JB, Douglas VP, et al. The scope of three-dimensional digital visualization systems in vitreoretinal surgery. Clin Ophthalmol Auckl NZ 2019;13:2093-6.
9. Tian W, Fan M, Zeng C, Liu Y, He D, Zhang Q. Telerobotic spinal surgery based on 5G network: the first 12 cases. Neurospine 2020;17:114-20.
10. Lacy AM, Bravo R, Otero-Piñeiro AM, et al. 5G-assisted telementored surgery. Br J Surg 2019;106:1576-9.
11. Agranat JS, Miller JB. 3D Surgical viewing systems in vitreoretinal surgery. Int Ophthalmol Clin 2020;60:17-23.
12. Jha AK. Time to get serious about pay for performance. JAMA 2013;309:347-8.
13. Bourcier T, Chammas J, Becmeur P-H, et al. Robot-assisted simulated cataract surgery. J Cataract Refract Surg 2017;43:552-7.
14. Bourcier T, Chammas J, Gaucher D, et al. Robot-assisted simulated strabismus surgery. Transl Vis Sci Technol 2019;8:26.
15. Chammas J, Sauer A, Pizzuto J, et al. Da Vinci Xi robot-assisted penetrating keratoplasty. Transl Vis Sci Technol 2017;6:21.
16. Tsirbas A, Mango C, Dutson E. Robotic ocular surgery. Br J Ophthalmol 2007;91:18-21.
17. Bourla DH, Hubschman JP, Culjat M, Tsirbas A, Gupta A, Schwartz SD. Feasibility study of intraocular robotic surgery with the da Vinci surgical system. Retina 2008;28:154-8.
18. Pandey SK, Sharma V. Robotics and ophthalmology: are we there yet? Indian J Ophthalmol 2019;67:988-94.
19. de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol 2018;29:248-53.
20. Molaei A, Abedloo E, de Smet MD, et al. Toward the art of robotic-assisted vitreoretinal surgery. J Ophthalmic Vis Res 2017;12:212-8.
21. Talcott KE, Adam MK, Sioufi K, et al. Comparison of a three-dimensional heads-up display surgical platform with a standard operating microscope for macular surgery. Ophthalmol Retina 2019;3:244-51.