A 30 year old woman with gradual vision loss OU
Digital Journal of Ophthalmology 2005
Volume 11, Number 15
August 15, 2005
Volume 11, Number 15
August 15, 2005
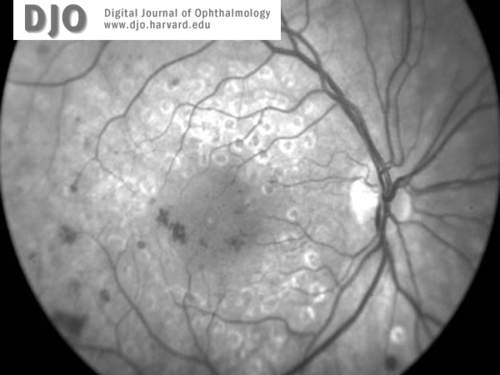
Figure 1a
Red free images of right (1a) and left (1b) maculae and discs at the time of presentation. Concentric grid laser scars and cystic edema are present in both eyes. There is no apparent neovascularization.
Red free images of right (1a) and left (1b) maculae and discs at the time of presentation. Concentric grid laser scars and cystic edema are present in both eyes. There is no apparent neovascularization.
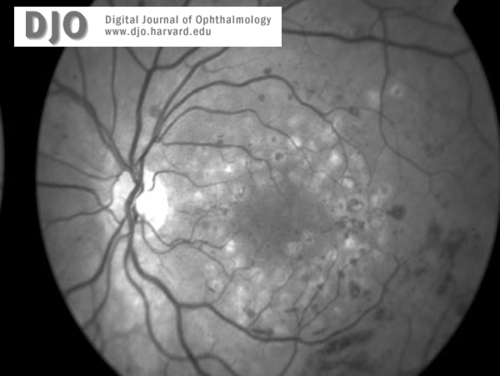
Figure 1b
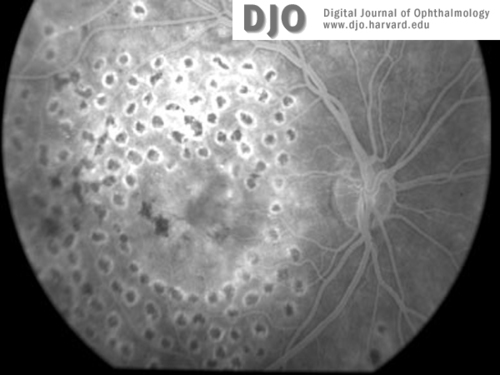
Figure 2a
Fluorescein image of the right (2a) and left (2b)maculae and discs at the time of presentation. Macular edema is evident in both eyes.
Fluorescein image of the right (2a) and left (2b)maculae and discs at the time of presentation. Macular edema is evident in both eyes.
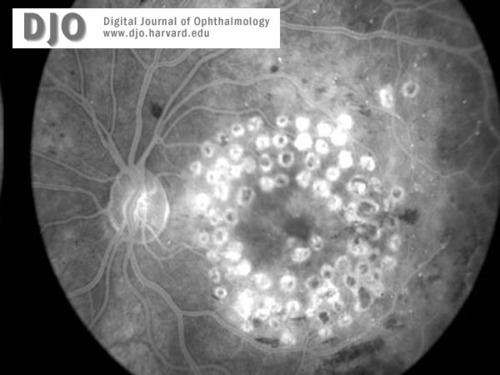
Figure 2b
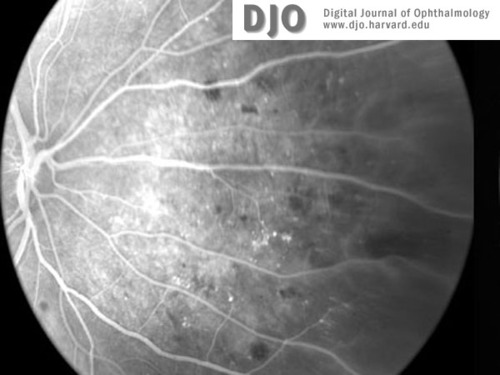
Figure 3a
Fluorescein image of right (3a) and left (3b) retinal peripheries at the time of presentation. Peripheral retinal ischemia without neovascularization is evident in both eyes.
Fluorescein image of right (3a) and left (3b) retinal peripheries at the time of presentation. Peripheral retinal ischemia without neovascularization is evident in both eyes.
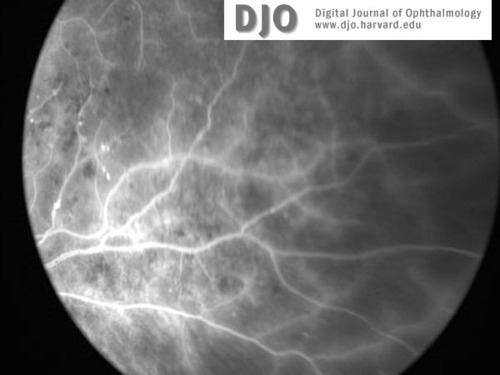
Figure3b
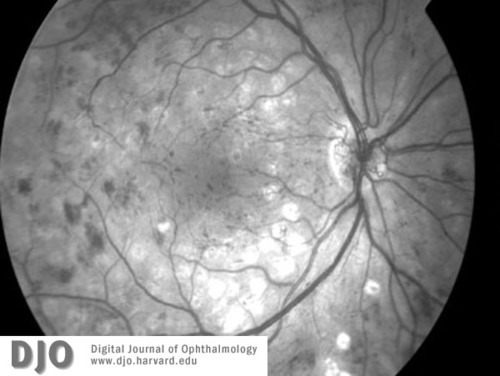
Figure 4a
Red-free image of right (4a) and left (4b)maculae and discs 3 months later, at the time of diagnosis with leukemia (CML). Proliferative retinopathy and one disc area of neovascularization at the disc is seen in both eyes. Extensive intraretinal hemorrhages are also seen.
Red-free image of right (4a) and left (4b)maculae and discs 3 months later, at the time of diagnosis with leukemia (CML). Proliferative retinopathy and one disc area of neovascularization at the disc is seen in both eyes. Extensive intraretinal hemorrhages are also seen.
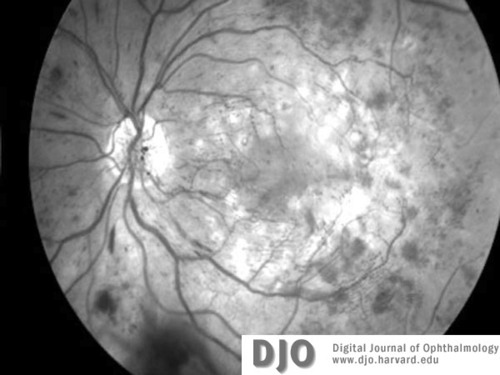
Figure 4b
Three months later she presented to her primary care provider complaining of fatigue and dehydration. Laboratory studies revealed a white blood cell count of 157,000 with blasts present on peripheral smear, a hematocrit of 30.9, and a platelet count of 346,000. A bone marrow aspiration was performed and cytogenetic studies revealed a reciprocal translocation between chromosomes 9 and 22 (Philadelphia chromosome), consistent with the diagnosis of chronic myelogenous leukemia (CML). A repeat dilated fundus exam at that time showed extensive intraretinal hemorrhages, neovascularization of both optic discs, and development of PDR with HRC (Figure 5, 6). There was severe ischemia of the peripheral retina in both eyes with widespread dropout of the peripheral retinal capillary bed (Figure 7, 8).
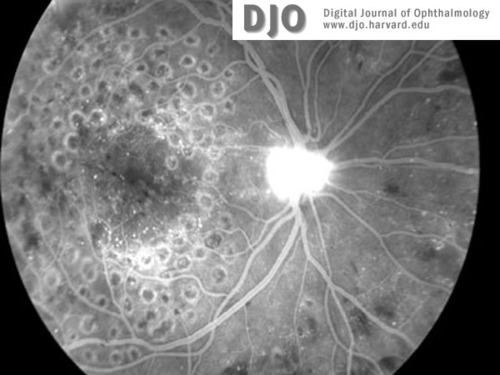
Figure 5a
Fluorescein image of right (5a) and left (5b) maculae at the time of diagnosis with CML. Neovascularization of both discs (with HRC) is evident. There is global capillary dropout encroaching on the left macula.
Fluorescein image of right (5a) and left (5b) maculae at the time of diagnosis with CML. Neovascularization of both discs (with HRC) is evident. There is global capillary dropout encroaching on the left macula.
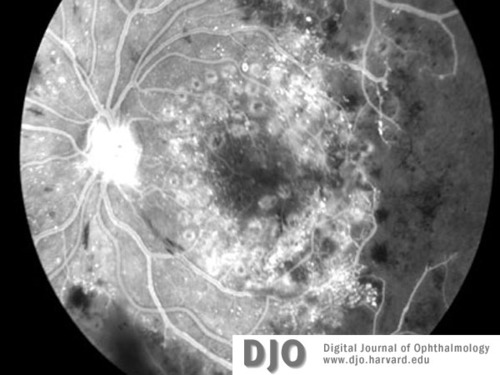
Figure 5b
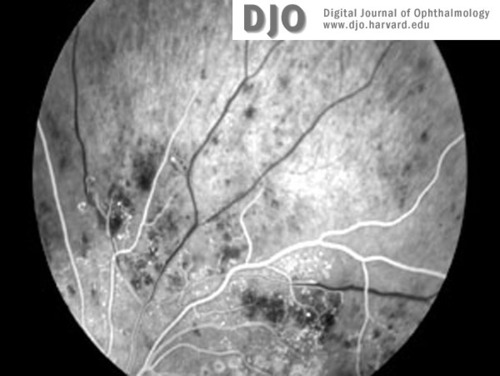
Figure 6a
Fluorescein images (a-c) of right and left retinal peripheries at the time of diagnosis with CML. Severe retinal ischemia with global infarction of the peripheral capillary bed is seen in both eyes. See also Figure 5b.
Fluorescein images (a-c) of right and left retinal peripheries at the time of diagnosis with CML. Severe retinal ischemia with global infarction of the peripheral capillary bed is seen in both eyes. See also Figure 5b.
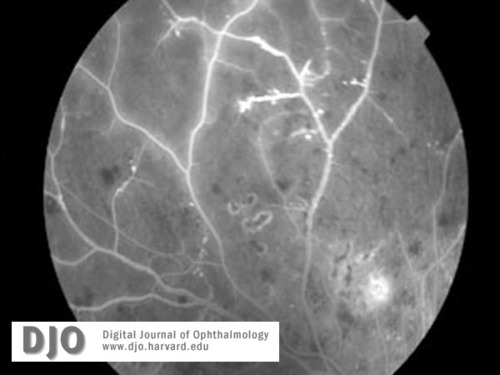
Figure 6b
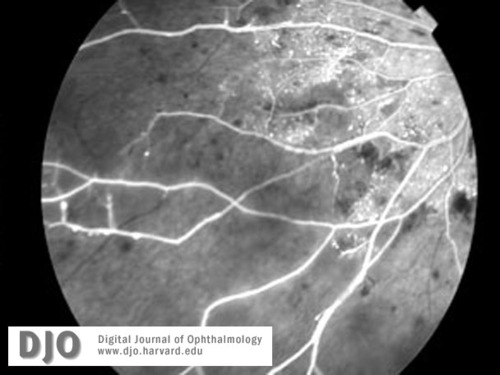
Figure 6c
The progression to bilateral PDR with HRC in only 3 months; however, is striking in this case. Melberg et al. described a patient with acute lymphocytic leukemia who progressed from background diabetic retinopathy to severe proliferative diabetic retinopathy over a period of six months (4). The authors concluded that the accelerated course of progression was attributable to moderate anemia related to the leukemia and its treatment. In our patient, there was also severe retinal ischemia; however, we propose an alternative hypothesis and mechanism for the accelerated progression to proliferative disease.
Leukoembolization of the retina with resulting ischemia from infarction of the retinal capillary bed is seen in numerous conditions manifesting as Purtscher’s-like retinopathy (5). The mechanism of microvascular occlusion in these diseases is generally believed to be leukocyte aggregation resulting from activation of complement factor C5a. We suggest that the unusually rapid progression of her disease was exacerbated by a similar mechanism, i.e. granulocyte aggregation with secondary microvascular occlusion due to severe leukocytosis. Aggregation of stimulated granulocytes has been proposed as a mechanism of microvascular occlusion, as well as pressure-dependent plugging of vessels and endothelial cell damage by toxic leukocyte products (6). This aggregation hypothesis is supported by evidence of increased blood viscosity and leukostasis in the vasculature of brains of patients who have died from leukemia (7). The clinical presentation of severe peripheral retinal ischemia in our patient may have been due in part to unrecognized leukoembolization of the retinal vasculature prior to her diagnosis of CML. The progressive retinal ischemia may have exacerbated the secretion of angiogenic factors in the diseased retina and led to accelerated development of neovascular disease.
Interestingly, recent studies have found that systemic blood plasma concentrations of angiogenic factors, including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are significantly increased in patients with CML, compared to healthy controls (8,9). Extravascular leakage of circulating VEGF and bFGF from incompetent vessels into the compromised retina may also act to increase the local tissue levels of these angiogenic factors and accelerate the clinical course of proliferative disease. Increased amounts of circulating VEGF, produced independently of underlying retinal ischemia in patients with CML, could also provide a synergistic mechanism for the rapid development of PDR with high risk characteristics observed in our patient.
2. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107:237-43.
3. ETDRS Treatment Group. Early Photocoagulation for Diabetic Retinopathy, ETDRS Report Number 9. Ophthalmology 1991;98:766-85.
4. Melberg NS, Grand MG, Rup D. The impact of acute lymphocytic leukemia on diabetic retinopathy. J Pediatr Hematol Oncol. 1995;17:81-84.
5. Chaum, E. (2000) Purtscher’s Retinopathy, In eMedicine, Ophthalmology. www.eMedicine.com.
6. Ernst E, Hammerschmidt DE, Bagge U, et al. Leukocytes and the risk of ischemic diseases. JAMA. 1987; 257:2318-24.
7. Phair JP, Anderson RE, Maniki H. The central nervous system in leukemia. Ann Intern Med. 1964;61:863.
8. Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000; 96: 2240-5.
9. Sillaber C, Mayerhofer M, Aichberger KJ, et al. Expression of angiogenic factors in chronic myeloid leukaemia: role of the bcr/abl oncogene, biochemical mechanisms, and potential clinical implications. Eur J Clin Invest. 2004; 34 Suppl 2:2-11.
