A 56-year-old man with a unilateral central scotoma
Digital Journal of Ophthalmology 2021
Volume 27, Number 3
September 27, 2021
Volume 27, Number 3
September 27, 2021
Download PDF
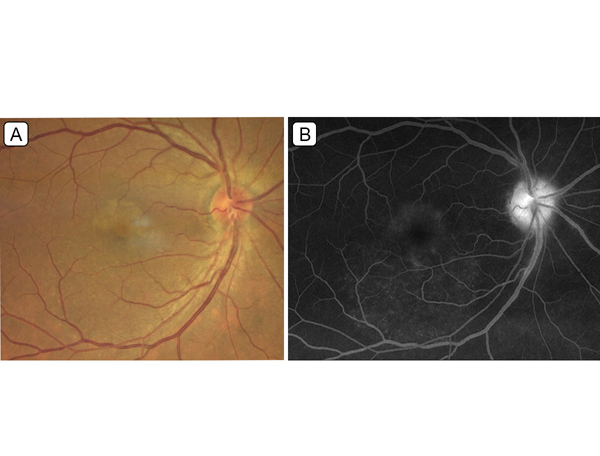
Figure 1.
A, Color fundus photograph of the right eye. B, Fluorescein angiogram of the right eye (5:09 minutes).
A, Color fundus photograph of the right eye. B, Fluorescein angiogram of the right eye (5:09 minutes).
Anti-treponemal antibodies (FTA-ABS) were drawn, and antibody absorption was positive, with a highly elevated rapid plasma reagin (RPR) titer of 1:1,024. The patient was then sent to our associated hospital for evaluation by the infectious disease service. Further workup included quantitative HIV (human immunodeficiency virus) antigen/antibody panel, HIV-1 RNA level, quantiFERON-TB Gold assay, anti-neutrophil cytoplasmic antibody panel, and angiotensin-converting enzyme levels, all of which were negative. Complete blood count with differential, comprehensive metabolic panel, and urinalysis were within normal limits. The team elected not to perform a lumbar puncture.
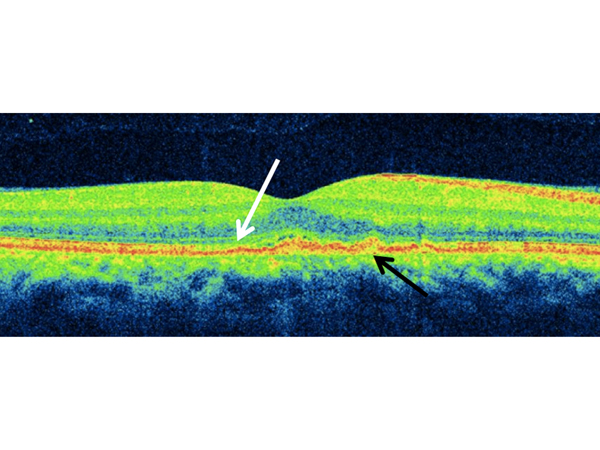
Figure 2.
Spectral domain optical coherence tomography (SD-OCT) of the right eye on presentation revealing small multifocal subretinal pigment epithelial (RPE) deposits (black arrow), with disruption of the ellipsoid zone (EZ) and photoreceptor layer (white arrow).
Spectral domain optical coherence tomography (SD-OCT) of the right eye on presentation revealing small multifocal subretinal pigment epithelial (RPE) deposits (black arrow), with disruption of the ellipsoid zone (EZ) and photoreceptor layer (white arrow).

Figure 3.
SD-OCT on 2-week follow-up after treatment showing partial reconstitution of EZ and reduction in sub-RPE deposits.
SD-OCT on 2-week follow-up after treatment showing partial reconstitution of EZ and reduction in sub-RPE deposits.
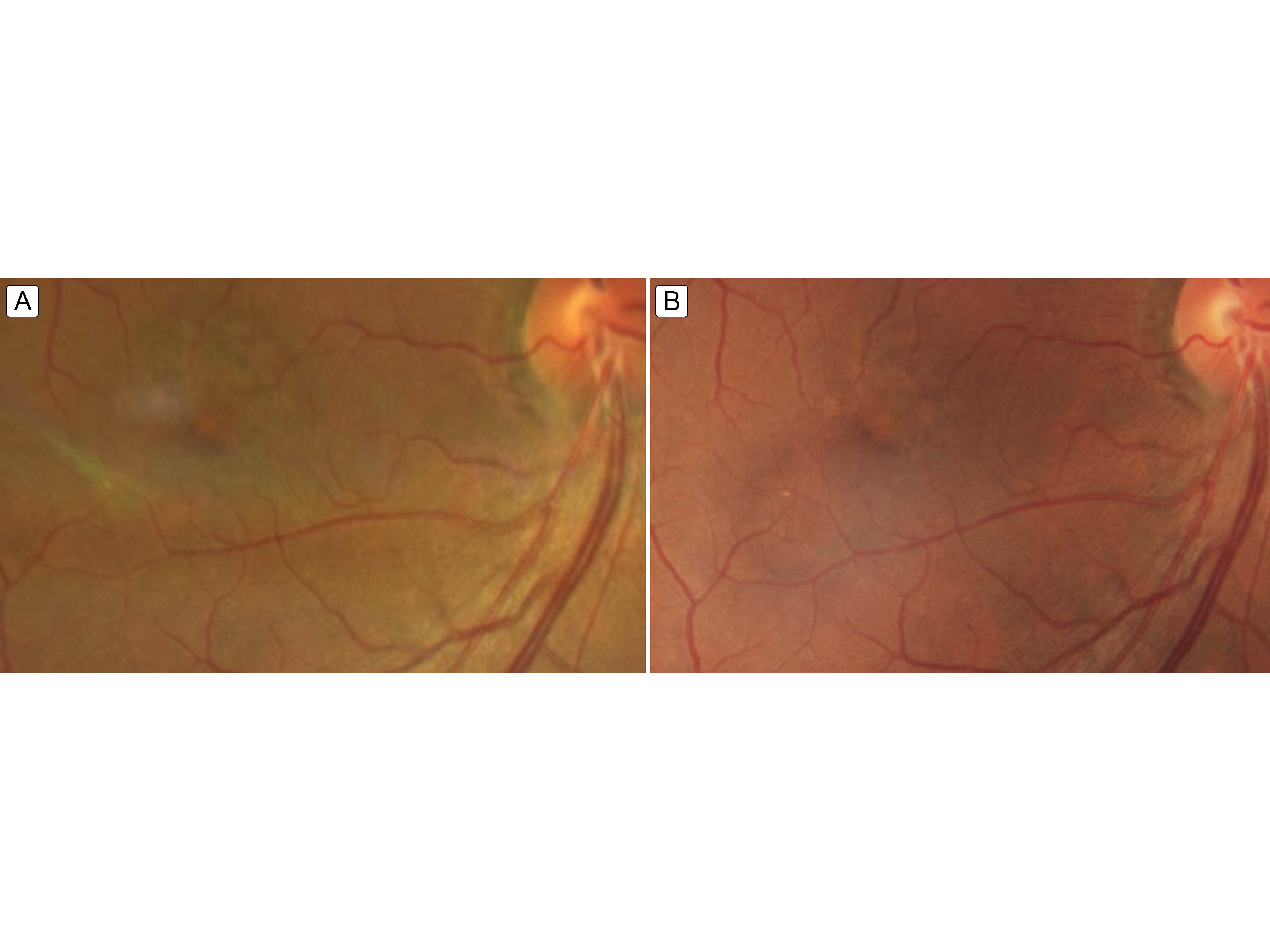
Figure 4.
Fundus image 2 weeks (A) and 6 weeks (B) after completing intravenous penicillin treatment showing ongoing but resolving mild foveal pigment mottling.
Fundus image 2 weeks (A) and 6 weeks (B) after completing intravenous penicillin treatment showing ongoing but resolving mild foveal pigment mottling.
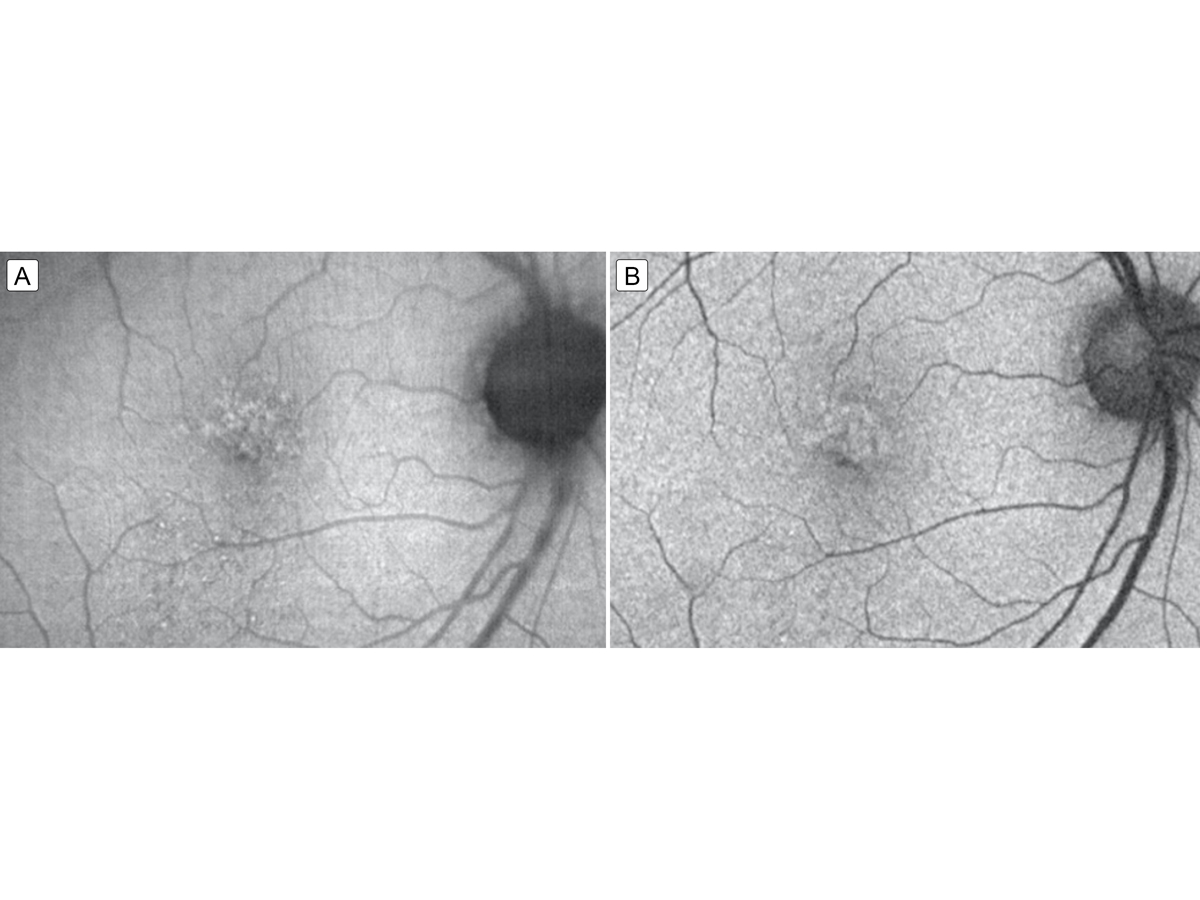
Figure 5.
Fundus autofluorescence 2 weeks (A) and 6 weeks (B) after completing intravenous penicillin treatment showing resolving punctate hyperfluorescent lesions that correspond to the resolving placoid lesion.
Fundus autofluorescence 2 weeks (A) and 6 weeks (B) after completing intravenous penicillin treatment showing resolving punctate hyperfluorescent lesions that correspond to the resolving placoid lesion.

Figure 6.
SD-OCT on 6-week follow up with resolution of sub-RPE deposits and mild residual sub-foveal disruption of photoreceptor outer segments.
SD-OCT on 6-week follow up with resolution of sub-RPE deposits and mild residual sub-foveal disruption of photoreceptor outer segments.
The differential diagnosis for this case includes viral retinitis, sarcoid, serpiginous retinopathy, serpiginous-like choroiditis, acute posterior multifocal placoid pigment epitheliopathy, acute macular neuroretinopathy, Vogt-Koyanagi-Harada syndrome, paraneoplastic retinopathy, and age-related macular degeneration. ASPPC can be distinguished from these conditions by its characteristic clinical and angiographic features, in particular, inflammation precisely delineated over a circular area of outer retina and inner choroid.(4) Importantly, ASPPC examination findings can overlap with various inflammatory ophthalmic conditions, and clinicians must first rule out infectious etiologies to avoid inappropriate treatment with systemic steroids. A study by Franco et al showed that antimicrobial treatment alone achieved complete ocular inflammatory recovery in patients with ASPPC, whereas a trial of corticosteroids provided no clinical benefit.(1)
The pathophysiology of ASPPC is not completely understood. One proposed mechanism is an inflammatory or immune-complex mediated reaction.(2) Studies have described an inflammatory origin at the level of the choroid-RPE-photoreceptor complex. Moll-Udina et al have proposed the choriocapillaries as the site of inflammation in ASPPC, because multimodal optical coherence tomography angiography (OCTA) demonstrates large areas of nonperfusion in this vascular layer.(9) This lack of blood flow can predispose the retina to further damage in the photoreceptor and RPE layers if left untreated. OCTA has been a useful tool to capture the reversible disruption of choriocapillaris flow in ASPPC.(10) These abnormalities may be transient upon diagnosis but typically resolve with early treatment.(10) First proposed by Gass et al and corroborated by multiple studies, T. pallidum is thought to enter the eye via choroidal circulation and most significantly affects the macula, where the choroidal vascular supply is the greatest.(3) Indocyanine green angiography has been a useful imaging modality, highlighting the choriocapillaris flow void and typically demonstrating hypofluorescence in the lesion sites.(11-13) Excellent functional outcome with outer retinal recovery is achievable if ASPPC is recognized and treated promptly with systemic penicillin therapy.
Hallmark imaging findings of ASPPC include FAF demonstrating localized hyperautofluorescence in the area of the placoid lesion secondary to sub-RPE deposits and inadequate phagocytic removal of outer segments.(3) These hyperautofluorescent areas on FAF colocalize with sub-RPE deposits seen on OCT; it has been suggested that this represents accumulation of lipofuscin at the level of the RPE-photoreceptor complex.(5) Other studies propose that these accumulations are collections of fibrin, platelets, and/or inflammatory cellular debris.(3) Our patient presented with discontinuity of the RPE, EZ, and photoreceptor layers; more severe findings in ASPPC include the accumulation of subretinal fluid and loss of external limiting membrane.(14) Shallow serous retinal detachment on OCT has also been described in patients with ASPPC.(3)
Prompt diagnosis with an initial screen of FTA-ABS, followed by non-treponemal RPR titers and HIV testing, are essential to guide treatment decisions and management in ASPPC.(1,4,9) The Centers for Disease Control and Prevention guidelines recommend that patients with ocular syphilis should undergo lumbar puncture with cerebrospinal fluid examination and be treated for neurosyphilis.(15) In our case, the admitting infectious disease team opted toward treating for neurosyphilis without lumbar puncture, because it would not have changed management. Up to 30%-60% of ocular syphilis patients may be coinfected with HIV.(1,4,9) Although HIV patients historically have been at higher risk of developing more severe forms of ocular syphilis, a study by Pichi et al demonstrated the presence of ASPPC in more than two-thirds of HIV negative, immunocompetent patients.(1,9) Previous studies have reported no differences in vision improvement after therapy with respect to HIV status. Our patient tested negative for HIV.
Several interventional and mechanistic studies have shed light on the pathogenesis of ASPPC. Some studies support an autoimmune etiology of the condition and cite higher levels of anti-beta 2 glycoprotein antibodies in patients with ASPPC, with the potential to cause focal choroidal thrombosis and photoreceptor damage.(1,2) Sahin et al reported that the use of methotrexate in ocular syphilis was beneficial as an adjuvant therapy to penicillin, causing decreased intraocular inflammation and cystoid macular edema.(16) In contrast, others have reported that long- and short-term corticosteroid use is associated with progression of ASPPC, suggesting that immune suppression may be a risk factor for ASPPC.(4,8,17) Thus, competing theories contend that ASPPC lesions may arise from a direct attack by T. pallidum as a result of reactivation from immunosuppression or, alternatively, may be a consequence of indirect immune-mediated hypersensitivity.(2) Prompt recognition and early treatment are essential in preventing and reversing vision loss in patients with ASPPC. Future studies with clinic-histopathologic correlation may help augment our understanding of the pathogenesis of ASPPC.(3)
2. Ormaechea MS, Hassan M, Nguyen QD, Schlaen A. Acute syphilitic posterior placoid chorioretinopathy: An infectious or autoimmune disease? Am J Ophthalmol Case Rep 2019;14:70-3.
3. Pichi F, Ciardella AP, Cunningham ET, Nucci P. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina 2014;34:373-84.
4. Eandi CM, Neri P, Adelman RA, Yannuzzi LA, Cunningham ET; International Syphilis Study Group. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina 2012;32:1915-41.
5. Davis JL. Ocular syphilis. Curr Opin Ophthalmol 2014;25:513-8.
6. Dailey K. Acute syphilitic posterior placoid chorioretinitis. Rev Optom, June 15, 2017. https://www.reviewofoptometry.com/article/ro0617-the-larry-alexander-resident-case-report-contest.
7. Huang C-Y, Kang EY-C, Chen K-J, Wang N-K. Acute syphilitic posterior placoid chorioretinopathy mimicking central serous chorioretinopathy: a case report. Taiwan J Ophthalmol 2018;8:176-8.
8. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology 1990;97:1288-97.
9. Moll-Udina A, Figueroa-Vercellino JP, Llorenç V, Miguel L, Adán A. Angiography and en face optical coherence tomography findings in acute syphilitic posterior placoid chorioretinopathy. Case Rep Ophthalmol 2019;10:165-71.
10. Tsui E, Gal-Or O, Ghadiali Q, Freund KB. Multimodal imaging adds new insights into acute syphilitic posterior placoid chorioretinitis. Retin Cases Brief Rep 2018;12 Suppl 1:S3-S8.
11. Bellmann C, Holz FG, Breitbart A, Völcker HE. Bilaterale akute syphilitische posteriore plakoide Chorioretinopathie (ASPPC). Angiographie- und Autofluoreszenz-Merkmale Ophthalmologe 1999;96:522-8.
12. Puech C, Gennai S, Pavese P, et al. Ocular manifestations of syphilis: recent cases over a 2.5-year period. Graefes Arch Clin Exp Ophthalmol 2010;248:1623-9.
13. Meira-Freitas D, Farah ME, Höfling-Lima AL, Aggio FB. Optical coherence tomography and indocyanine green angiography findings in acute syphilitic posterior placoid choroidopathy: case report. Arq Bras Oftalmol 2009;72:832-5.
14. Stephens JD, Dunn JP. Progressively worsening vision in one eye and recent loss of vision in the other marks a patient whose history includes syphilis. Rev Ophthalmol, January 2016. https://www.reviewofophthalmology.com/article/january-2016-wills-eye-resident-case-series.
15. Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States 2015. Atlanta GUHaHS, CDC; 2016. http://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm.
16. Sahin O, Ziaei A. Clinical and laboratory characteristics of ocular syphilis, co-infection, and therapy response. Clin Ophthalmol Auckl NZ. 2016;10:13-28.
17. Erol N, Topbas S. Acute syphilitic posterior placoid chorioretinitis after an intravitreal triamcinolone acetonide injection. Acta Ophthalmol Scand 2006;84:435.