A 54-year-old man with bilateral symmetrical circular corneal opacities
Digital Journal of Ophthalmology 2020
Volume 26, Number 2
June 21, 2020
DOI: 10.5693/djo.03.2019.12.001
Volume 26, Number 2
June 21, 2020
DOI: 10.5693/djo.03.2019.12.001
Download PDF
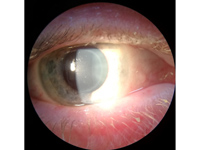
Figure 1
Slit-lamp photograph of the right eye showing intrastromal corneal circular opacity.
Slit-lamp photograph of the right eye showing intrastromal corneal circular opacity.

Figure 2
Optical coherence tomography image demonstrating the stromal opacity sparing the epithelium and endothelium.
Optical coherence tomography image demonstrating the stromal opacity sparing the epithelium and endothelium.
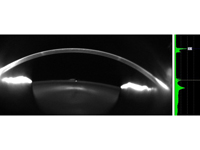
Figure 3
Scheimpflug Pentacam HR image demonstrating the stromal opacity sparing the epithelium and endothelium.
Scheimpflug Pentacam HR image demonstrating the stromal opacity sparing the epithelium and endothelium.
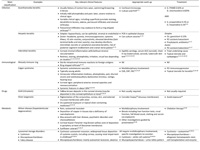
Table 1
Differential diagnosis of circular/ring-shaped corneal opacities
Differential diagnosis of circular/ring-shaped corneal opacities

Table 1 (cont.)
The absence of corneal vascularization and symmetrical appearance may point more toward a degeneration or dystrophy, such as a phenotypic variation of a stromal dystrophy, with perhaps the most similar being Schnyder corneal dystrophy.(7) The degenerations of crocodile shagreen and Vogt’s limbal girdle differ in appearance, as does the Hudson-Stähli line in iron deposition, Stocker’s line in keratoconus, and posterior embryotoxon.
Other alternative considerations are immunological and infective responses such as Coats white ring, Wessely immune ring, Gram-negative rods, fungi, herpes simplex/zoster, and Acanthamoeba. However, none of these fit the history and clinical features of the present case. Other differentials include immunoglobulin deposition as part of a multiple myeloma, which was excluded in our case.(8)
Corneal opacities may be secondary to a wide array of etiologies, including trauma, infection, or inflammation. They may also result from drug deposition, metabolic disorders, and corneal dystrophies or degenerations. It is important in such rare presentations to take an accurate history and to arrange appropriate investigations. The perfect circular shape and isolation in the midperipheral cornea suggest that this case likely represents deposition from an unknown material.
2. Ascher KW. Ungewöhnliche Hornhautringe [Unusual corneal ring]. Dtsch Ophthal Ges 1963;65:44-6.
3. Roberts WH, Wolter JR. Ocular chrysiasis. AMA Arch Ophthalmol 1956;56:48-52.
4. Spencer WH, Garron LK, Contreras F, Hayes TL, Lai C. Endogenous and exogenous ocular and systemic silver deposition. Trans Ophthalmol Soc U K 1980;100:171-8.
5. Melles GR, de Sera JP, Eggink CA, Cruysberg JR, Binder PS. Bilateral, anterior stromal ring opacity of the cornea. Br J Ophthalmol 1998;82:522-5.
6. Patel DV. Systemic associations of corneal deposits: a review and photographic guide. Clin Exp Ophthalmol 2017;45:14-23.
7. Weiss JS, Moller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies—edition 2. Cornea 2015;34:117-59.
8. Miller KH, Green WR, Stark WJ, Wells HA, Mendelsohn G, Kanhofer H. Immunoprotein deposition in the cornea. Ophthalmology 1980;87:944-50.
9. Robaei D, Carnt N, Minassian DC, Dart JK. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: risk factors, outcomes, and summary of the literature. Ophthalmology 2015;122:17-24.
10. Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol 2009;148:487-99 e2.
11. Denniston AKO, Murray PI. Oxford Handbook of Ophthalmology. 4th ed. Oxford: Oxford University Press; 2018.
12. Jackson TL. Moorfields Manual of Ophthalmology. 2nd ed. New Delhi: JP Medical Ltd; 2014.
13. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology 2017;124:1678-89.
14. Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol 2016;44:824-37.
15. Bowling B. Kanski’s Clinical ophthalmology: a systematic approach. 8th ed. Philadelphia: Elsevier; 2016.
16. Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol 2009;54:226-34.
17. Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: safety and efficacy of a new treatment for herpes simplex keratitis. Curr Eye Res 2012;37:654-60.
18. Calvo CM, Sikder S, Mamalis N, Mifflin MD. Linear interstitial keratitis: a distinct clinical entity revisited. Cornea 2012;31:1500-503.
19. Knox CM, Holsclaw DS. Interstitial keratitis. Int Ophthalmol Clin 1998;38:183-95.
20. Kauffmann DJ, Wormser GP. Ocular Lyme disease: case report and review of the literature. Br J Ophthalmol 1990;74:325-7.
21. Wessely K. About anaphylactic phenomena of the cornea. München Med Wehascher. 1911;58:1713.
22. Morawiecki J. Antigen-antibody precipitation phenomena in the living cornea. Ophthalologica 1956;132:236-43.
23. Qiao GL, O’Donnell H, Yeung SN, Iovieno A. Case of bilateral Wessely rings in a contact lens wearer. Can J Ophthalmol 2019;54:e182-e3.
24. Orsoni JG, Zavota L, Pellistri I, Piazza F, Cimino L. Cogan syndrome. Cornea 2002;21:356-9.
25. Espinoza GM, Prost A. Cogan’s syndrome and other ocular vasculitides. Curr Rheumatol Rep 2015;17:24.
26. Iliescu DA, Timaru CM, Batras M, De Simone A, Stefan C. Cogan’s Syndrome. Rom J Ophthalmol 2015;59:6-13.
27. Kessel A, Vadasz Z, Toubi E. Cogan syndrome—pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 2014;13:351-4.
28. Mohsenin A, Huang JJ. Ocular manifestations of systemic inflammatory diseases. Conn Med 2012;76:533-44.
29. Greco A, Gallo A, Fusconi M, et al. Cogan’s syndrome: an autoimmune inner ear disease. Autoimmun Rev 2013;12:396-400.
30. Kincaid MC, Green WR, Hoover RE, Schenck PH. Ocular chrysiasis. Arch Ophthalmol 1982;100:1791-4.
31. McCormick SA, DiBartolomeo AG, Raju VK, Schwab IR. Ocular chrysiasis. Ophthalmology 1985;92:1432-5.
32. Loewenstein A. Argyrosis of Conjunctiva, Cornea and Tear-Sac. Br J Ophthalmol 1941;25:360-69.
33. Wilkes JW. Argyrosis of the cornea and conjunctiva. J Tn State Med Assoc 1953;46:11-3.
34. Moss AP, Sugar A, Hargett NA, Atkin A, Wolkstein M, Rosenman KD. The ocular manifestations and functional effects of occupational argyrosis. Arch Ophthalmol 1979;97:906-8.
35. Tendler I, Pulitzer MP, Roggli V, Abramson DH, Marr BP. Ocular argyrosis mimicking conjunctival melanoma. Cornea 2017;36:747-8.
36. Tso MO, Fine BS, Thorpe HE. Kayser-Fleischer ring and associated cataract in Wilson’s disease. Am J Ophthalmol 1975;79:479-88.
37. Herron BE. Wilson’s disease (hepatolenticular degeneration). Ophthalmic Semin 1976;1:63-9.
38. Ryan A, Nevitt SJ, Tuohy O, Cook P. Biomarkers for diagnosis of Wilson’s disease. Cochrane Database Syst Rev 2019;2019:
39. Poujois A, Woimant F. Wilson’s disease: A 2017 update. Clin Res Hepatol Gastroenterol 2018;42:512-20.
40. Naik MP, Sethi HS, Dabas S. Ocular cystinosis: Rarity redefined. Indian J Ophthalmol 2019;67:1158-9.
41. Biswas S, Gaviria M, Malheiro L, Marques JP, Giordano V, Liang H. Latest clinical approaches in the ocular management of cystinosis: a review of current practice and opinion from the Ophthalmology Cystinosis Forum. Ophthalmol Ther 2018;7:307-22.
42. Del Longo A, Piozzi E, Schweizer F. Ocular features in mucopolysaccharidosis: diagnosis and treatment. Ital J Pediatr 2018;44:125.
43. Sornalingam K, Javed A, Aslam T, Sergouniotis P, Jones S, Ghosh A, Ashworth J. Variability in the ocular phenotype in mucopolysaccharidosis. Br J Ophthalmol 2019;103:504-10.
44. Sivley MD, Benjamin WJ. Fabry keratopathy: manifestations and changes over time. Br J Ophthalmol 2019 Oct 30. doi: 10.1136/bjophthalmol-2019-314906. Epub ahead of print.
45. Michaud L. Longitudinal study on ocular manifestations in a cohort of patients with Fabry disease. PLoS One 2019;14:e0213329.
46. Davies G, Dymock I, Harry J, Williams R. Deposition of melanin and iron in ocular structures in haemochromatosis. Br J Ophthalmol 1972;56:338-42.
47. Hudson JR. Ocular findings in haemochromatosis. Br J Ophthalmol. 1953;37:242-6.
48. Ustaoglu M, Solmaz N, Baser B, Kurtulgan HK, Onder F. Ocular and genetic characteristics observed in two cases of fish-eye disease. Cornea 2019;38:379-83.
49. Viestenz A, Schlotzer-Schrehardt U, Hofmann-Rummelt C, Seitz B, Kuchle M. Histopathology of corneal changes in lecithin-cholesterol acyltransferase deficiency. Cornea 2002;21:834-7.
50. Cogan DG, Kruth HS, Datilis MB, Martin N. Corneal opacity in LCAT disease. Cornea 1992;11:595-9.
51. Bron AJ. Corneal changes in the dislipoproteinaemias. Cornea 1989;8:135-40.
52. Lewis RA, Falls HF, Troyer DO. Ocular manifestations of hypercupremia associated with multiple myeloma. Arch Ophthalmol 1975;93:1050-3.
53. Silkiss RZ, Pomerleau D, Sorenson A, Vastine D, Crawford JB. Corneal cupremia in multiple myeloma: a clinicopathologic correlation. Arch Ophthalmol 2008;126:1005-6.
54. Rodrigues MM, Krachmer JH, Miller SD, Newsome DA. Posterior corneal crystalline deposits in benign monoclonal gammopathy: a clinicopathologic case report. Arch Ophthalmol 1979;97:124-8.
55. de Alba Campomanes AG, Rutar T, Crawford JB, Seiff S, Goodman D, Grenert J. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea 2009;28:1081-4.
56. Martin NF, Kincaid MC, Stark WJ, et al. Ocular copper deposition associated with pulmonary carcinoma, IgG monoclonal gammopathy and hypercupremia: a clinicopathologic correlation. Ophthalmology 1983;90:110-6.
57. Gjone E, Norum KR. Corneal arcus and hyperlipoproteinaemia. Lancet 1968;2:359.
58. Rifkind BM. Corneal arcus and hyperlipoproteinaemia. Surv Ophthalmol 1972;16:295-304.
59. Rennie CA, Chowdhury S, Khan J, et al. The prevalence and associated features of posterior embryotoxon in the general ophthalmic clinic. Eye (Lond) 2005;19:396-9.
60. Ozeki H, Shirai S, Majima A, Sano M, Ikeda K. Clinical evaluation of posterior embryotoxon in one institution. Jpn J Ophthalmol 1997;41:422-5.
61. Coats G. Two Cases Showing a small superficial opaque white ring in the cornea. Trans Ophthal Soc UK 1912;32:53-6.