A 57-year-old man with leukocytosis and sphenoid sinus disease
Digital Journal of Ophthalmology 2020
Volume 26, Number 2
April 24, 2020
DOI: 10.5693/djo.03.2019.09.003
Volume 26, Number 2
April 24, 2020
DOI: 10.5693/djo.03.2019.09.003
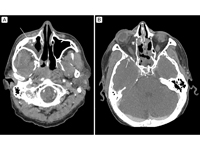
Figure 1
Thin slice, contrast-enhanced computed tomography (CT) in an axial plane at two levels show opacification of the right sphenoid and maxillary sinuses, indicated with arrows. Figure 1A demonstrates significant proptosis of the right eye, with early postseptal infiltration in the form of inflammatory stranding of the retro-orbital fatty tissue with reactive osteitis. Figure 1B, a lower axial cut, better demonstrates the opacification of the right maxillary sinus.
Thin slice, contrast-enhanced computed tomography (CT) in an axial plane at two levels show opacification of the right sphenoid and maxillary sinuses, indicated with arrows. Figure 1A demonstrates significant proptosis of the right eye, with early postseptal infiltration in the form of inflammatory stranding of the retro-orbital fatty tissue with reactive osteitis. Figure 1B, a lower axial cut, better demonstrates the opacification of the right maxillary sinus.
Three days after his initial admission, the proptosis worsened, and the IOP increased to 45 mm Hg in the right eye. The following day, the left eye had new proptosis, decreased visual acuity (20/60), and an increase in IOP to 22 mm Hg. Extraocular motility of the left eye appeared restricted in all gazes. Anterior examination revealed proptosis, ptosis, and chemosis. Dilated fundus examination remained normal.
Five days after admission, visual acuity in the left eye also progressed to no light perception. His pupil was fixed at 5 mm and nonreactive. Extraocular motility was nonexistent. He developed neck stiffness, left lower extremity and forehead numbness, and intermittent confusion.
Initial bacterial blood cultures grew Enterobacter cloacae; fungal blood cultures had no growth. Four days after admission, lumbar puncture revealed leukocytosis, with increased polymorphonucleocytes and monocytes despite negative viral, bacterial, fungal, and amoeba cultures of the cerebral spinal fluid.
Six days after admission, and 5 days after an urgent sinus sphenoidotomy, surgical pathology revealed nonseptate hyphae consistent with zygomycetes of the Mucor family. Repeat endoscopic sinus debridement noted no focal necrotic tissue.
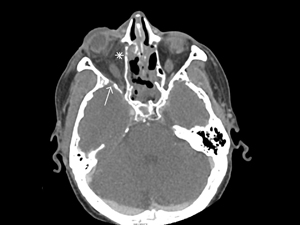
Figure 2
Contrast-enhanced CT imaging in an axial plane demonstrating postseptal infiltration (asterisk) and reactive osteitis (arrow).
Contrast-enhanced CT imaging in an axial plane demonstrating postseptal infiltration (asterisk) and reactive osteitis (arrow).
His IOP spikes on day 2 in the right eye and day 3 in the left eye were treated with brimonidine 0.2%, dorzolamide 2%, and timolol 0.5% each twice daily in the right eye.
Three days after admission, an urgent sinus sphenoidotomy showed no necrotic tissue, purulence, or fungal debris. He was transitioned to vancomycin, cefepime, metronidazole, and amphotericin. On post-admission day 11, he became diaphoretic, obtunded, and hypotensive, with no response to resuscitation. He was transitioned to comfort care and died that day.
Although rare, RCOM in immunocompetent individuals has been reported. An analysis of the literature of mucormycosis in immunocompetent, otherwise healthy individuals found 81 of 212 patients (38.2%) presenting with RCOM.(7) The present report reveals the need to have a high index of suspicion for RCOM in immunocompetent patients, especially if they are clinically deteriorating despite appropriate antibiotics.
Early diagnosis, removal or reversal of risk factors, prompt antifungals, and surgical debridement of devitalized tissue optimize RCOM outcomes.(8) Additionally, early evaluation and diagnosis of sinus mucormycosis helps prevent orbital invasion. Recognizing early findings such as fever, facial pain with swelling, nasal mucosal ulceration/necrosis, sinusitis, decreased visual acuity, and headache assists in timely diagnosis.(6,9) Amphotericin B is associated with increased response rates and survival.(8) Prompt amphotericin B initiation is recommended because a delay of >6 days is associated with increased mortality.(10) Early surgical debridement of necrotic tissue is fundamental. Necrotic tissue can decrease antifungal penetration, decreasing drug effectiveness.(11)
Tissue cultures are not necessarily reliable, because samples often fail to grow Mucor. Debridement biopsies help identify Mucor on tissue preparations using direct microscopy. Improved outcomes are associated with therapeutic decisions made based on frozen tissue sections.(12) Furthermore, Mucor tissue infections may manifest without a black necrotic eschar, as in our case. Mucormycosis must be considered irrespective of tissue presentation.
Mortality rates of RCOM range from 30% to 69%.(4) Despite following standard practice guidelines, our patient’s prognosis was poor. We hypothesize that intranasal steroids may have predisposed our immunocompetent patient. Local steroids likely suppressed immune responses, rendering the nasal mucosa susceptible to fungal colonization. An intensive literature search found no other reports of local steroid therapy with RCOM.
Rapidly deteriorating RCOM may occur in both immunocompromised and immunocompetent individuals. A high index of suspicion must be held in patients with signs and symptoms suspicious for mucormycosis sinusitis, especially if an immunocompetent individual is on intranasal steroids. Prompt administration of antifungal medications and debridement of necrotic tissues are recommended in all patients with RCOM.
Literature Search
PubMed was searched on July 21, 2016, without date restriction, for English-language results, using the following terms: rhino-orbital-cerebral mucormycosis, mucor, and immunocompetent.
2. Montone KT. Differential diagnosis of necrotizing sinonasal lesions. Arch Pathol Lab Med 2015;139:1508-14.
3. Montone KT, LiVolsi VA, Lanza DC, et al. In situ hybridization for specific fungal organisms in acute invasive fungal rhinosinusitis. Am J Clin Pathol 2011;135:190-9.
4. Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect 2004;10 Suppl 1:31-47.
5. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634-53.
6. Wali U, Balkhair A, Al-mujaini A. Cerebro-rhino orbital mucormycosis: an update. J Infect Public Health 2012;5:116-26.
7. Mignogna MD, Fortuna G, Leuci S, et al. Mucormycosis in immunocompetent patients: a case-series of patients with maxillary sinus involvement and a critical review of the literature. Int J Infect Dis 2011;15:e533-40.
8. Sun HY, Singh N. Mucormycosis: its contemporary face and management strategies. Lancet Infect Dis 2011;11:301-11.
9. Yohai RA, Bullock JD, Aziz AA, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol 1994;39:3-22.
10. Chamilos G, Lewis RE, Kontoyiannis DP. Delayed amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2000;47:503-9.
11. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005;18:556-69.
12. Van burik JA, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis 2006;42:e61-5.