A 24-year-old woman with sudden-onset, unilateral vision loss
Digital Journal of Ophthalmology 2019
Volume 25, Number 4
November 17, 2019
DOI: 10.5693/djo.03.2019.09.004
Volume 25, Number 4
November 17, 2019
DOI: 10.5693/djo.03.2019.09.004
Download PDF
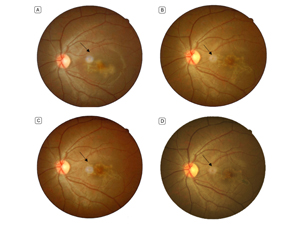
Figure 1
Fundus photographs. A, 10 days after disease onset: a darkish, serpiginous lesion is seen surrounded by a pale halo. B, 4th week after disease onset: the lesion is larger and more established, although visual acuity improved. C, 7th week after disease onset: the lesion is becoming smaller. D, 10th month after disease onset: the overall size of the lesion is smaller, but the honeycomb pattern is more distinguished. The black arrows indicate optical artifacts caused by light reflection.
Fundus photographs. A, 10 days after disease onset: a darkish, serpiginous lesion is seen surrounded by a pale halo. B, 4th week after disease onset: the lesion is larger and more established, although visual acuity improved. C, 7th week after disease onset: the lesion is becoming smaller. D, 10th month after disease onset: the overall size of the lesion is smaller, but the honeycomb pattern is more distinguished. The black arrows indicate optical artifacts caused by light reflection.
Fluorescein angiography (FA) 10 days after disease onset revealed a hypofluorescent patch with hyperfluorescent margins (1/2 disc diameters), smaller than the funduscopic lesion, along with a small nodule of adjacent hyperfluorescence corresponding to leakage (1/4 disc diameters). The margins of the lesion showed leakage and staining in the late phases of the angiogram (Figure 4A).
Testing also included a purified protein derivative (PPD) skin test due to the serpiginoid pattern of the lesion, the leakage observed on FA, and tuberculosis being endemic in the region. The patient had a positive PPD test (15 mm of induration).

Figure 2
Optical coherence tomography. Ten days after disease onset of the disease the right eye (A) appeared normal; the left eye (B) had dome-shaped heterogeneous hyper-reflective depositions in the outer retina, beneath the fovea, with disruption of the outer retinal layers, and a hyporeflective area (blue circle) attributable to fluid and hyper-reflectivity of the outer nuclear layer temporal to the fovea. In the 4th (C) and 7th (D) weeks of follow-up the undulations and irregularities in the retinal pigment epithelium layer (RPE) became less visible; the hyper-reflective depositions and fluid largely subsided. The junction of the inner and out segments seemed to reappear in the 7th week. In the 10th month (E) the outer retina appeared partially restored, and the nodular areas of thickened RPE were resolving. In the 19th month (F) the outer retinal abnormalities largely resolved.
Optical coherence tomography. Ten days after disease onset of the disease the right eye (A) appeared normal; the left eye (B) had dome-shaped heterogeneous hyper-reflective depositions in the outer retina, beneath the fovea, with disruption of the outer retinal layers, and a hyporeflective area (blue circle) attributable to fluid and hyper-reflectivity of the outer nuclear layer temporal to the fovea. In the 4th (C) and 7th (D) weeks of follow-up the undulations and irregularities in the retinal pigment epithelium layer (RPE) became less visible; the hyper-reflective depositions and fluid largely subsided. The junction of the inner and out segments seemed to reappear in the 7th week. In the 10th month (E) the outer retina appeared partially restored, and the nodular areas of thickened RPE were resolving. In the 19th month (F) the outer retinal abnormalities largely resolved.
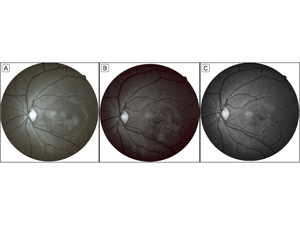
Figure 3
Red-free imaging. A nonhomogenous hyper-reflective patch was seen in the 1st (A) and 4th (B) week of follow-up. The margins became sharper and better demarcated, and the center appeared more hypo-reflective in the 4th week. In the 10th month of follow-up (C), the lesion became smaller and borders better delineated. The number and intensity of central hypo-reflective areas increased and led to a more distinct honey-comb pattern.
Red-free imaging. A nonhomogenous hyper-reflective patch was seen in the 1st (A) and 4th (B) week of follow-up. The margins became sharper and better demarcated, and the center appeared more hypo-reflective in the 4th week. In the 10th month of follow-up (C), the lesion became smaller and borders better delineated. The number and intensity of central hypo-reflective areas increased and led to a more distinct honey-comb pattern.
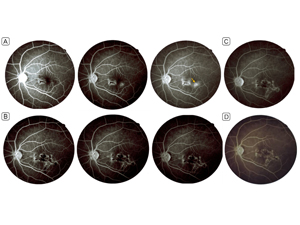
Figure 4
Fluorescein angiography. Ten days after disease onset, early- to late-phase images (A) show a macular hypofluorescent patch, with hyperfluorescent margins and leakage in late phases and a small nearby leaking nodule (yellow arrow). In the 4th week of follow-up, the early- to late-phase images (B) show the leaking nodule merged with the rest of the lesion, acquiring a serpiginous morphology. In the 7th week (C), no leakage is evident; no change was observed in the size or the angiographic pattern from the 4th week, and the lesion evolved into a honey-comb pattern. At 19 months’ follow-up (D) the lesion appears stable, with no significant change in size/pattern or signs of reactivation.
Fluorescein angiography. Ten days after disease onset, early- to late-phase images (A) show a macular hypofluorescent patch, with hyperfluorescent margins and leakage in late phases and a small nearby leaking nodule (yellow arrow). In the 4th week of follow-up, the early- to late-phase images (B) show the leaking nodule merged with the rest of the lesion, acquiring a serpiginous morphology. In the 7th week (C), no leakage is evident; no change was observed in the size or the angiographic pattern from the 4th week, and the lesion evolved into a honey-comb pattern. At 19 months’ follow-up (D) the lesion appears stable, with no significant change in size/pattern or signs of reactivation.
At week 7, visual acuity was 10/10 in both eyes. The patient still reported some metamorphopsia in the left eye. At 10 months’ and 19 months’ follow-up, visual acuity was still 10/10, but the patient reported some improvement in metamorphopsia. Fundus examination revealed healing with scar formation in the periphery of the lesion (Figure 1D). Macular OCT showed that the dome-shaped hyper-reflective depositions and retinal pigment epithelium (RPE) irregularities had resolved to a large extent (Figure 2E). The patient had not started the recommended anti-tuberculosis therapy.
We also considered acute idiopathic maculopathy (AIM) and acute retinal pigment epithelitis (ARPE), because they can also appear with sudden-onset unilateral vision loss in young patients who experience full visual recovery.(6,7) They can also be preceded by a flulike illness, which did not occur in our patient. Additionally, the fundus findings and imaging for our patient were not entirely consistent with AIM, in which circumscribed grayish pigmentary changes are usually seen in the macular region in the active phase, becoming more like a bull’s eye on recovery.(7,8) Also, OCT findings in AIM include hyper-reflective thickening of the outer retina and RPE in the foveal region, sometimes accompanied by hyper-reflective exudation and hyporeflective fluid in the subretinal space and neurosensory detachment. On FA, AIM patients usually exhibit an early irregularly speckled hyperfluourescence and hypofluorescence in the area of the active lesion that may be followed by dye pooling in the region of subretinal fluid, if present, in the late phase.(7,8) The lesion in our patient showed expansion on FA in the 4th week and was not compatible with angiographic features of AIM. Our case was also not fully similar to usual cases of ARPE in terms of the morphology of the lesion and FA findings.
The active lesion in ARPE is usually described as an area of pigment stippling in the macula, with a surrounding hypopigmented halo. Our case did have a hypopigmented halo in the early stages.
In ARPE the majority of changes observable on OCT are reported to happen in the outer retina and include RPE thickening, irregular (dome-shaped) depositions, disruption of IS/OS junction, and anterior dislocation of retinal layers in the subfoveal region.(6,10) The OCT findings of our case were similar to prior studies in these respects. However, in our case, a hyporeflective space was seen below the external limiting membrane in the subfoveal area that was probably fluid (Figure 2B).
Prior case reports of ARPE have also shown improvement of OCT findings in follow-up examinations (1-36 months); for example, significant reduction in RPE irregularities/thickness and reappearance of the IS/OS junction. In the majority of cases, the resolution of OCT abnormalities did not start until 1-2 months of disease onset. In some cases, abnormalities lasted up to 12 months.(6,10) Fairly consistent with prior case reports, the recovery of the outer retina was observed on OCT during the first month of follow-up in our case, and a near complete recovery was not seen until 10 months.
The majority of prior reports on Krill disease have shown transmission defects (hyperfluorescence), with no leakage on FA.(6,10) Yet instances of no noticeable change have also been reported.(10) Some reports have described the angiographic pattern as a hypofluorescent area surrounded by a hyperfluorescent rim.(13-15) In our case, the initial FA could be considered similar to what previous reports have noted as an area of hypofluorescence with early hyperfluorescence at the margins; however, the leakage and staining of the rims of the initial lesion and the subretinal leakage adjacent to the lesion in our case were unlike previously reported cases. Follow-up FA imaging showed a honeycomblike pattern of hyper- and hypofluorescence in the area of the inactive lesion, and the leakage and staining were no longer observed (Figure 3B-C). The lesions in ARPE usually become less distinct over time and leave an area of subtle pigment mottling; this was also not the case in our patient, because the lesion progressed to areas of scarring that were evident both on FA and fundus examination.
Previous studies have reported a good visual acuity recovery in most of the ARPE cases within 6-12 weeks.(6,10,11) Recovery was also seen in our patient, and visual acuity of 10/10 returned within 25 days. The vision quality still had not improved to the level of the fellow eye by final follow-up at 19 months (persistent micropsia and metamorphopsia).
The bigger size and serpiginous pattern of the lesion in our case were unlike previously reported cases of Krill disease. The observed fluid in OCT and the leakage area on FA were indicative of choroidal involvement, although serous leakage has been reported in Krill patients on disruption of the RPE. The main points of similarity of our case with Krill cases were the rapid recovery of visual acuity and the pattern of outer retinal involvement in OCT imaging.
Serpiginous choroidopathies, particularly macular serpiginous choroiditis and atypical SLC, can be considered a diagnosis. A majority of prior cases, however, have reported that SLC has multifocal lesions and does not invade the foveal region, even in the presence of macular involvement, early in its course.(16) Although the morphology of the lesion in our case was serpiginous type, there was foveal involvement from the beginning, and the lesion remained solitary at the final visit, an observation more in favor of macular serpiginous choroiditis.
OCT in patients with serpiginous choroiditis and SLC shows outer nuclear layer (ONL) hyper-reflectivity (small or punctiform spots within the ONL), choriocapillaris, point-like hyper-reflections, and outer retinal tabulation. Some OCT signs are observed significantly more often in patients with tuberculosis-associated SLC, including vitreal hyper-reflective spots, intraretinal edema, sub-RPE drusenoid deposits, and choroidal granulomas.(17,18) The disruption of the outer retinal layers has been reported in some cases.(3,17) In our patient, the outer retina and RPE were primarily involved. Additionally, corresponding to the leaking nodule, an ONL hyper-reflectivity was observed in the temporal foveal region, changing to some RPE irregularities at 4-7 weeks and clearing by the final follow-up (Figure 2F). The site of ONL involvement, however, lacked the usually reported punctate points of hyper-reflectivity. We also did have evidence of subretinal fluid and increased choroidal thickness. Overall, compared to our case, abnormalities in OCT images of active choroiditis lesions were more severe.(17,18)
The change in OCT imaging over time in our patient is noteworthy. Studies investigating the effects of antituberculosis and anti-inflammatory treatment in typical macular serpiginous choroiditis and SLC cases reported attenuation and permanent scarring of the outer retinal layers with inactivation of the lesion over a period of 6-12 months.(1,19) In our patient, the OCT abnormalities were largely resolved during the first month, without treatment; this is unusual for a serpiginous lesion, which generally takes longer to heal.
In terms of the FA observations, the shape of the lesion, the staining and leakage at margins of the active lesion, and the expansion toward the leakage point is consistent with macular serpiginous choroiditis and SLC.(4,17) However, the active lesion was not at the margins of the established lesion, as is commonly observed in serpiginous choroidopathies or SLC.
Any serpiginous choroiditis involving the fovea has an unfavorable visual outcome.(4) Lesions remain for several months and leave dense scar tissue when they finally become inactive.(4,17,20) In our case, there was full visual recovery by day 25 of follow-up, and the lesion had become inactive. Overall, the most distinguishing features of this case were fast recovery of visual acuity, resolution of OCT abnormalities, and inactivation of lesions without any treatment, which feature is not typical macular serpiginous choroiditis or SLC.
Although in this case an inflammatory self-limiting process seems the most likely explanation for our patient’s clinical course, a tuberculosis-related pathophysiology cannot be ruled out. Both SLC and macular serpiginous choroiditis can be induced by tuberculosis; however, with the absence of a multifocal lesion and/or vitritis, that is, signs more commonly observed in SLC, macular serpiginous choroiditis seems more likely. Studies have shown that an isolated positive PPD test has a very low predictive value in ascertaining the role of tuberculosis in countries endemic for the disease.(21) Therefore, the results of a PPD test should be interpreted cautiously to avoid unnecessary treatments.
In a typical patient with ARPE, the primary site of involvement is the outer retina and the RPE. The retinal lesions usually show pigment stippling in the macular area, with a surrounding hypopigmented halo. On OCT, the lesion appears as an area of dome-shaped hyper-reflective depositions in the subfoveal area with disruption of the outer retinal layers; on FA, it shows a transmission defect, with no leakage.(6,9,13) The crucial point in diagnosing ARPE is the spontaneous and near-complete recovery of visual acuity and resolution of retinal lesions within a short timeframe, usually 1-3 months. On the other hand, in typical patients with serpiginous choroidopathies, the primary site of involvement is the choroid. These patients usually have retinal lesions with a serpiginous appearance that may be either bilateral or unilateral and solitary or multifocal. They show abnormalities in both the choroid and the overlying outer retina on OCT, including retinal edema and choroidal pointlike hyper-reflections. On FA, retinal lesions appear as hypofluorescent areas that stain or leak at the margins in the late phases. They usually require treatment with anti-inflammatory or anti-tuberculosis drugs (SLC) to hasten the progression and speed up the process of inactivation, which can take up to several months. Their natural course can vary, however, and in the absence of treatment they tend to relapse and extend their scar. With involvement of the fovea, the visual prognosis is usually poor.(2,3)
Although the morphology in this case resembles that of macular serpiginous choroidopathy, the relatively fast recovery of function and structure made that diagnosis less likely. The presence of choroidal involvement and subretinal fluid, however, was atypical for other diagnoses, including Krill disease and AIM. Our case represents an atypical form of macular serpiginous choroidopathy, with a benign course.
Acknowledgments
The authors thank Dr. Alireza Khodabande, Faculty of Ophthalmology, Farabi Eye Hospital, Tehran University of Medical Sciences, for his insights on this case.
2. Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol 2013;58:203-32.
3. Vasconcelos-Santos DV, Rao PK, Davies JB, Sohn EH, Rao NA. Clinical features of tuberculous serpiginouslike choroiditis in contrast to classic serpiginous choroiditis. Arch Ophthalmol 2010;128:853-8.
4. Gan WL, Jones NP. Serpiginous-like choroiditis as a marker for tuberculosis in a non-endemic area. Br J Ophthalmol 2013;97:644-7.
5. Golchet PR, Jampol LM, Wilson D, Yannuzzi LA, Ober M, Stroh E. Persistent placoid maculopathy: a new clinical entity. Trans Am Ophthalmol Soc 2006;104:108-20.
6. Puche N, Offret O, Bernard JA, Behar-Cohen F. A case of acute retinal pigment epithelitis: spectral domain optical coherence tomography time course and physiopathologic hypothesis. Clin Ophthalmol 2010;4:1029-33.
7. Jung CS, Payne JF, Bergstrom CS, et al. Multimodality diagnostic imaging in unilateral acute idiopathic maculopathy. Arch Ophthalmol 2012;130:50-6.
8. de la Fuente MA, Cuadrado R. Unilateral acute idiopathic maculopathy: angiography, optical coherence tomography and microperimetry findings. J Ophthalmic Inflamm Infect 2011;1:125-7.
9. Blanco-Rivera C, Campos-Garcia S. Acute retinal pigment epitheliitis: a case report [in Spanish]. Arch Soc Esp Oftalmol 2007;82:451-3.
10. Cho HJ, Han SY, Cho SW, et al. Acute retinal pigment epitheliitis: spectral-domain optical coherence tomography findings in 18 cases. Invest Ophthalmol Vis Sci 2014;55:3314-9.
11. Lo Giudice G, Crepaldi V, Giulio Catania A, Galan A. Retinal epithelitis as ocular side effects of oral biphosphonates. Clin Case Rep Rev 2017;3(8). DOI: 10.15761/CCRR.1000355.
12. Friedman MW. Bilateral recurrent acute retinal pigment epitheliitis. Am J Ophthalmol 1975;79:567-70.
13. Gilhotra JS, Gilhotra AK, Holdaway IM, Donaldson ML. Acute retinal pigment epitheliitis associated with intravenous bisphosphonate. Br J Ophthalmol 2006;90:798-9.
14. Aydo?an T, Güney E, Akçay B?, Bozkurt TK, Ünlü C, Ergin A. Acute retinal pigment epitheliitis: spectral domain optical coherence tomography, fluorescein angiography, and autofluorescence findings. Case Rep Med 2015;2015:149497.
15. Blanco-Rivera C, Campos-García S. Epitelitis retiniana aguda: a propósito de un caso. Arch Soc Esp Oftalmol 2007;82:451-3.
16. Gupta A, Bansal R, Gupta V, Sharma A. Fundus autofluorescence in serpiginouslike choroiditis. Retina 2012;32:814-25.
17. Zarei M, Mohsenzadeh N, Roohipoor R, Riazi-Esfahani H. Successful treatment of tubercular multifocal serpiginous-like choroiditis without use of anti-inflammatory drugs: a case report with multimodal imaging. J Curr Ophthalmol 2019;31:229-33.
18. Wang XN, You QS, Zhao HY, Peng XY. Optical coherence tomography features of tuberculous serpiginous-like choroiditis and serpiginous choroiditis. Biomed Environ Sci 2018;31:327-34.
19. Bansal R, Kulkarni P, Gupta A, Gupta V, Dogra MR. High-resolution spectral domain optical coherence tomography and fundus autofluorescence correlation in tubercular serpiginouslike choroiditis. J Ophthalmic Inflamm Infect 2011;1:157-63.
20. Belakbir T, Nourdine B, Omar L. Serpiginous-like choroiditis as sign of intraocular tuberculosis. J Fr Ophtalmol 2019:42:932-4.