A 76-year-old woman with progressive right-sided proptosis, blepharoptosis, vision loss, and ophthalmoplegia
Digital Journal of Ophthalmology 2019
Volume 25, Number 3
August 25, 2019
Volume 25, Number 3
August 25, 2019
Download PDF

Figure 1
Clinical photographs of nine cardinal positions of gaze in patient with right eye exhibiting complete ophthalmoplegia in all directions of gaze. Due to complete right-sided ptosis, the upper eyelid was manually supported during photography.
Clinical photographs of nine cardinal positions of gaze in patient with right eye exhibiting complete ophthalmoplegia in all directions of gaze. Due to complete right-sided ptosis, the upper eyelid was manually supported during photography.
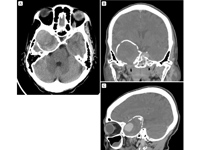
Figure 2
Computed tomography (CT) imaging demonstrating a giant cavernous carotid aneurysm. Axial CT (A) shows a calcified aneurysmal lining with displacement of the right temporal lobe; coronal (B) and sagittal (C) CT images demonstrate the large size of the giant aneurysm.
Computed tomography (CT) imaging demonstrating a giant cavernous carotid aneurysm. Axial CT (A) shows a calcified aneurysmal lining with displacement of the right temporal lobe; coronal (B) and sagittal (C) CT images demonstrate the large size of the giant aneurysm.
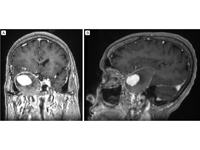
Figure 3
T1-weighted coronal (A) and sagittal (B) post-contrast magnetic resonance imaging (MRI) demonstrating a giant cavernous carotid aneurysm with partial thrombosis of the peripheral aneurysmal sac.
T1-weighted coronal (A) and sagittal (B) post-contrast magnetic resonance imaging (MRI) demonstrating a giant cavernous carotid aneurysm with partial thrombosis of the peripheral aneurysmal sac.
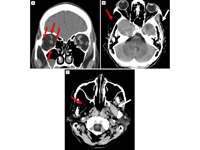
Figure 4
CT imaging. A, Coronal view showing right-sided atrophic extraocular muscles (red arrows). B, Axial view showing atrophy of the right temporalis muscle (red arrow) compared to normal muscle (white arrow). C, Axial view, showing atrophy of the right pterygoid muscles (red arrow) compared to normal (white arrow).
CT imaging. A, Coronal view showing right-sided atrophic extraocular muscles (red arrows). B, Axial view showing atrophy of the right temporalis muscle (red arrow) compared to normal muscle (white arrow). C, Axial view, showing atrophy of the right pterygoid muscles (red arrow) compared to normal (white arrow).
Epidemiologically, diplopia and orbital pain are the most common clinical symptoms, although partial ophthalmoplegia (with the sixth cranial nerve being most commonly affected because of to its location adjacent to the internal carotid artery within the cavernous sinus) and ptosis are the most common clinical signs.(1,4) Our patient exhibited additional ocular signs attributed to the aneurysm, including complete ophthalmoplegia, proptosis, and optic neuropathy. These signs are relatively rare presentations of a giant CCA. The largest case series of 206 CCAs of all sizes found that 18% of patients with CCAs exhibited a cavernous sinus syndrome involving cranial nerves 3, 4, and 6 in varying degrees, whereas a second series of giant CCA found that only 16% exhibited complete ophthalmoplegia.(1,4) Studies have shown that only 7% of CCAs exhibit proptosis, which is thought to be due to both laxity of the atrophied extraocular muscles allowing forward motion of the globe and also increased dural sinus congestion and venous pressure.(4) Notably, complete third nerve palsy, which our patient had, can also result in up to three millimeters of proptosis due to resultant extraocular muscle laxity. In our case, wasting of the extraocular muscles was evident on imaging studies as a result of the long-standing lesion. Although decreased vision in these cases can result from varying etiologies, such as corneal hypesthesia and keratopathy, compressive optic neuropathy is a rare cause of vision loss, as was the case in our patient.(1)
The patient also exhibited evidence of unilateral trigeminal neuropathy. The fifth cranial nerve supplies both sensory and motor innervation to facial structures. The first and second branch travel through the cavernous sinus, and the third branch exits the skull base through the foramen ovale prior to entering the cavernous sinus. Large cavernous sinus lesions, such as our patient’s aneurysm, exert sufficient mass effect to impinge on the third branch of the fifth cranial nerve prior to exiting the skull base. Our patient experienced corneal thinning and scarring suggestive of neurotrophic cornea as well as a right jaw thrust from unilateral pterygoid muscle atrophy and temporalis muscle wasting, indicating compressive neuropathy of the motor branch of the fifth cranial nerve. Muscle paralysis of the pterygoid and temporalis muscles from compressive CCA is a rare clinical sign, present in only 4% of cases and resulting from compression of the trigeminal ganglion in Meckel’s cave.(4)
In our case, the patient initially sought medical care as a result of seizurelike activity. While intracranial aneurysms rarely cause seizurelike activity, case reports of internal carotid aneurysms causing epileptiform seizures do exist.(5,6) To date, existing literature suggests that intracranial aneurysms are more likely to be epileptogenic if they are in direct contact with anterior temporomedial brain structures.(6) Indeed, CT and MRI imaging in our case showed displacement of temporal lobe parenchyma by the aneurysm, which lends support to the belief that the aneurysm may have led to the patient’s seizurelike symptoms.
Prior studies have found that giant CCA are more common in women and in advanced age.(1) While many CCA are idiopathic, these lesions may arise as a result of connective tissue disease, neoplasm, or vasculitis.(7) Specific subtypes of CCA result from trauma or infection, although these distinct entities each warrant unique evaluation and treatment strategies.(8,9) Given the non-life-threatening nature of these lesions, many warrant clinical observation initially. Studies have shown that untreated lesions may spontaneously regress, and that mild symptoms can spontaneously improve.(1) However, orbital pain or symptomatic diplopia may persuade some to consider neurosurgical intervention, as studies have shown that intervention is associated with a higher rate of resolution of CCA-related pain.(1) Notably, treatment has not been found to reliably forecast improvement of diplopia, although shorter duration of ophthalmoplegia is typically associated with a better chance of recovery.(1) Current treatment options for unruptured aneurysms include aneurysmal sac clamping, ligation, balloon occlusion of the proximal internal carotid artery, and most recently, flow diversion therapy.(2,10,11)
2. Ambekar S, Madhugiri V, Sharma M, Cuellar H, Nanda A. Evolution of management strategies for cavernous carotid aneurysms: a review. World Neurosurg 2014;82:1077-85.
3. Wiebers DO, Whisnant JP, Huston J, et al; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362(9378):103-10.
4. Hahn CD, Nicolle DA, Lownie SP, Drake CG. Giant cavernous carotid aneurysms: clinical presentation in fifty-seven cases. J Neuroophthalmol 2000;20:253-8.
5. Ellamushi H, Thorne L, Kitchen N. Unruptured cerebral aneurysms causing seizure disorder (report of two cases ). Seizure 1999;8:310-2.
6. Hänggi D, Winkler PA, Steiger H-J. Primary epileptogenic unruptured intracranial aneurysms: incidence and effect of treatment on epilepsy. Neurosurgery 2010;66:1161-5.
7. Miller N, Walsh FB, Hoyt WF. Walsh and Hoyt’s Clinical Neuro-Ophthalmology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
8. Bavinzski G, Killer M, Knosp E, Ferraz-Leite H, Gruber A, Richling B. False aneurysms of the intracavernous carotid artery—report of 7 cases. Acta Neurochir (Wien) 1997;139:37-43.
9. Glaiberman CB, Towbin RB, Boal DKB. Giant mycotic aneurysm of the internal carotid artery in a child: endovascular treatment. Pediatr Radiol 2003;33:211-5.
10. Abla AA, Lawton MT. Current treatment strategies for cavernous internal carotid artery aneurysms. World Neurosurg 2014;82:994-5.
11. Van Rooij WJ, Sluzewski M. Endovascular treatment of large and giant aneurysms. AJNR Am J Neuroradiol 2009;30:12-8.