An 11-month-old girl with a retinal detachment
Digital Journal of Ophthalmology 2019
Volume 25, Number 2
May 11, 2019
DOI: 10.5693/djo.03.2019.02.004
Volume 25, Number 2
May 11, 2019
DOI: 10.5693/djo.03.2019.02.004
Download PDF
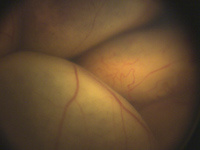
Figure 1
Fundus photograph showing vascular abnormalities in the retina with a yellow, bullous retinal detachment.
Fundus photograph showing vascular abnormalities in the retina with a yellow, bullous retinal detachment.
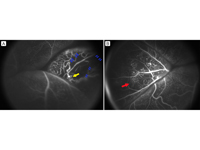
Figure 2
Fluorescein angiogram. A, Aneurysmal dilatations (yellow arrow) and capillary dropout (blue arrows). B, Telangiectatic vessels (red arrow).
Fluorescein angiogram. A, Aneurysmal dilatations (yellow arrow) and capillary dropout (blue arrows). B, Telangiectatic vessels (red arrow).
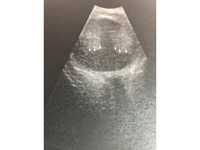
Figure 3
Figure 3. B-scan ultrasound showing an intraocular, hyperechoic mass representative of an exudative retinal detachment. Subretinal areas are hypoechoic (arrows), which goes against calcification, a feature associated with retinoblastoma and appearing as bright hyperechoic lesions.
Figure 3. B-scan ultrasound showing an intraocular, hyperechoic mass representative of an exudative retinal detachment. Subretinal areas are hypoechoic (arrows), which goes against calcification, a feature associated with retinoblastoma and appearing as bright hyperechoic lesions.
Video 1
Sluggish dynamics of the red blood cells traveling along the dilated retinal vessels and further highlights of the anomalous retinal vasculature.
Sluggish dynamics of the red blood cells traveling along the dilated retinal vessels and further highlights of the anomalous retinal vasculature.
A reduction in subretinal fluid was noted 3 months after laser treatment. At the final follow-up, 7 months after the procedure, the child could not fixate with this eye. A white cataract in the affected eye prevented visualization of the fundus. However, intraocular pressure remained normal, and there was no iris rubeosis. Current treatment for the affected eye is atropine 1% eye drops once a day for comfort. The right eye remains normal.
Retinoblastoma is an important differential consideration for leukocoria with a mass, because it is the most common primary ocular tumor in children. Other pediatric ocular tumors, such as choroidal melanoma, choroidal hemangioma, and medulloepithelioma are also important diagnoses. Leukocoria is also commonly seen in congenital cataracts.
The etiologies for retinal detachment in children differ from those in adults. A large proportion of pediatric retinal detachments are congenital in nature and include rare hereditary vitreoretinal conditions, such as familial exudative vitreoretinopathy, incontinentia pigmenti, Stickler syndrome, X-linked retinoschisis, Marfan syndrome, and Norrie disease.
In infants, developmental pathologies, such as persistent fetal vasculature and colobomas may be associated with retinal detachment, and retinopathy of prematurity is an important cause of retinal detachment in premature babies.
Retinal detachment may also occur because of trauma, inflammation, and infection; however, these usually present with more clues in the presenting history and have other signs of systemic involvement.
Coats disease is an ophthalmic condition classically presenting with reduced visual acuity, strabismus, or leukocoria.(2) It is a nonhereditary condition that manifests as an exudative process characterized by abnormal changes in retinal vasculature. The disease process involves intra- and subretinal exudation of fluids, which in later stages can lead to retinal detachment. The fundus is typically observed with telangiectasia, “light bulb” aneurysms, and intra- and subretinal exudates.(3,4) There appears to be no reported racial or ethnic predilection.(5) Additionally, it is unilateral in 80%-95% of cases.
The most widely used classification system for Coats’ disease was proposed by Shields et al in 2001.(1) Stage 1 refers to eyes that only have telangiectasia; stage 2, to eyes with exudation in the extrafoveal (2A) or subfoveal (2B) locations. In stage 3 eyes there is partial (3A) or total (3B) exudative detachment; if this is accompanied by glaucoma, it is classified as stage 4. Finally, stage 5 is observed with a complete detachment of the retina, which commonly presents with cataract and phthisis, and this can lead to complete blindness.(1)
Treatment of Coats disease is based on disease severity. Mild cases are managed conservatively. More advanced stages involving exudates and retinal or subretinal fluid are managed with laser photocoagulation or cryotherapy to abnormal vascular regions. The combination of these treatments is effective in preventing progression to neovascular glaucoma or phthisis in most patients, thus preserving the globe. In stage 3B Coats disease the aim of treatment is to prevent rubeosis through partial reattachment; thus, unrecordable visual acuity after treatment does not imply an unsuccessful outcome. There is also a role for anti-VEGF agents such as bevacizumab in early-stage macula-involving Coats disease; however, the safety profile in children is unclear.
The majority of patients presenting with Coats disease are male (75%) and usually present in the first decade of life, with the mean age of diagnosis at 5 years.(5) The mean age of diagnosis of retinoblastoma in children is 15-24 months, with no sex predilection.(6) Hence, while the diagnostic findings for Coats disease are typical with this case, the age and sex of the patient are atypical.
Retinoblastoma and Coats disease commonly mimic each another. Shields et al appraised 150 cases of Coats disease and established that the diagnosis was correct in only 64 cases (41%). The mistaken diagnoses included retinoblastoma in 43 cases (27%), retinal detachment in 12 (8%) and retinal hemorrhage in 7 (4%).(5) Conversely, when the same authors analyzed 604 cases with lesions simulating retinoblastoma, they found that Coats disease was the most common cause of pseudoretinoblastoma (40%).(7)
In earlier stages of Coats disease, fundus examination alone is enough to differentiate Coats disease from retinoblastoma: typically the lesion in Coats disease has a yellow-colored appearance, whereas in retinoblastoma it has a chalky whitish-gray appearance (see Shields J and Shields C. J AAPOS 2011;15:e7: Abstract 026). However, when retinal detachment with subretinal exudation and dilated retinal vessels coexist, even an expert may have difficulty differentiating Coats disease from retinoblastoma by ophthalmoscopy.
Over the last three decades, there has been a significant improvement in the detection of Coats disease. Fluorescein angiography is critical in documenting classic findings to establish the diagnosis, because it highlights anomalies in the retinal vasculature. Computed tomography (CT) and magnetic resonance imaging have also proved to be extremely helpful in aiding the diagnosis of Coats disease.(8) However, they cannot always be relied on for differentiating Coats from retinoblastoma. Sherman et al reported that CT imaging was unable to differentiate between unilateral noncalcifying retinoblastoma in 2 children with advanced Coats’ disease.(9) This limitation was emphasized by Haik et al’s report of similar findings in 14 patients with Coats disease.(10) However, it should be noted that any form of imaging involving radiation is contraindicated in children where retinoblastoma remains a suspicion, because they could carry a germline mutation in all their cells, predisposing them to secondary tumors.
Retinoblastoma can be treated with a combination of chemotherapy, transpupillary thermotherapy, cryotherapy, and brachytherapy; in severe cases, enucleation may be necessary. Although extraocular invasion and systemic spread are now rare in developed countries, this cancer can still lead to significant morbidity and mortality.
In the past, in cases where retinoblastoma could not be definitively excluded, ophthalmologists opted to enucleate an eye associated with a poor prognosis. This is vital to prevent a delay in diagnosis and definitive treatment of a potentially lethal condition. Inevitably, this approach also involved enucleating eyes with advanced Coats disease.(1) Thus, establishing a correct diagnosis early is essential for proper treatment.
2. Bowling B, Kanski JJ. Kanski’s Clinical Ophthalmology: A Systematic Approach. 8th ed. Edinburgh Elsevier; 2015.
3. McGettrick P.M., Loeffler KU. Bilateral Coats’ disease in an infant (a clinical, angiographic, light and electron microscopic study). Eye (Lond) 1987;1:136-45.
4. Theodossiadis G.P. Some clinical, fluorescein-angiographic, and therapeutic-aspects of Coats’ disease. J Pediatr Ophthalmol Strabismus 1979;16:257-62.
5. Shields JA, Shields CL, Honavar SG, et al. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol 2001;131: 561-71.
6. Dimaras H, Kimani K, Dimba EA, et al. Retinoblastoma. Lancet 2012. 379(9824):1436-46.
7. Shields C, Schoenberg E, Kocher K, Shukla S, Kaliki S, Shields J. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases. Ophthalmology 2013;120:311-6.
8. Shields JA, Parsons HM, Shields CL, et al. Lesions simulating retinoblastoma. J Pediatr Ophthalmol Strabismus 1991;28:338-40.
9. Sherman J, McLean I, Brallier D. Coats’ disease: CT-pathologic correlation in two cases. Radiology 1983;146:77-8.
10. Haik B, Saint Louis L, Smith M, et al. Magnetic resonance imaging in the evaluation of leukocoria. Ophthalmology 1985;92:1143-52.