|
|
Bilateral uveal effusions in a 23-year-old man
Digital Journal of Ophthalmology 2018
Volume 24, Number 2
May 22, 2018
DOI: 10.5693/djo.03.2018.03.001
|
Printer Friendly
|
|
|



Karen W. Jeng-Miller, MD, MPH | Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts Eric D. Gaier, MD, PhD | Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts Angela V. Turalba, MD | Department of Ophthalmology, Atrius Health, Boston, Massachusetts
|
|
|
| History |
| A 23-year-old white man with a past medical history of seasonal allergies and no prior ocular history presented emergently to the Massachusetts Eye and Ear Emergency Ward with acute, bilateral blurry vision of one day’s duration that had worsened overnight. He reported epiphora in both eyes and head pressure without headache. He described intermittent scintillations in both eyes of white and blue color. The patient reported taking a single dose of a “muscle relaxer” 10 days prior; he was unsure of the name of the drug. He denied any other new medications. He also reported an isolated episode of diarrhea on the day his blurry vision started. He denied eye pain, floaters, trauma, diplopia, fevers, rashes, recent travel, insect bites, myalgias, joint pain, dysuria, hematuria, melena, hematochezia, or shortness of breath. He cited occasional marijuana usage but no other recreational drug use. |
|
| Examination |
| On examination, his visual acuity was counting fingers in both eyes, improving with pinhole testing to 20/150 in the right eye and 20/125 in the left eye. His pupils were miotic and minimally reactive; there was no relative afferent pupillary defect. Intraocular pressure (IOP) was 54 mm Hg by Goldman applanation tonometry in each eye. Anterior segment examination revealed microcystic corneal edema and shallow anterior chambers in both eyes, with no signs of anterior chamber inflammation. There was no conjunctival injection or heterochromia of the iris. Gonioscopy demonstrated absence of angle structures in all four quadrants in each eye. Posterior segment examination of both eyes revealed normal optic nerves with cup-to-disc ratios of 0.1 and peripheral uveal effusions in all retinal quadrants. The maculae and vessels were within normal limits. |
|
| Ancillary Testing |
| Biometry testing revealed an axial length of 22.43 mm in the right eye and 22.26 mm in the left eye as measured by LENSTAR LS 900 Biometer (Haag-Streit USA). His anterior chamber depth was recorded at 2.06 mm in the right eye and 2.03 mm in the left eye. B-scan ultrasonography revealed uveal effusions without ocular masses (Figure 1) and ultrasound biomicroscopy (UBM) confirmed shallow anterior chambers with uveal effusions in both eyes (Figure 2A). |
|
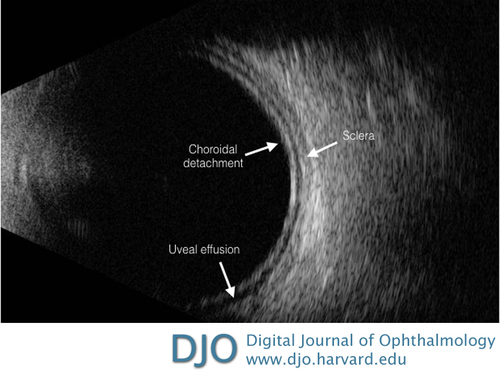
Figure 1
B-scan ultrasound of the left eye showing uveal effusion and no evidence of an intraocular mass. The same findings were present in the right eye.
|
|
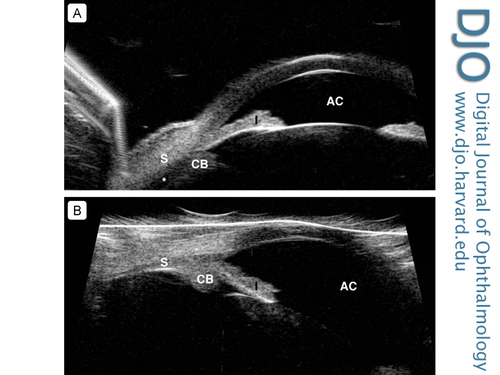
Figure 2
A, Ultrasound biomicroscopy (UBM) of the left eye showing evidence of peripheral uveal effusion (asterisk) and shallow anterior chamber (AC). The same findings were present in the right eye. B, UBM of the left eye demonstrating resolution of the peripheral uveal effusions with deepening of the anterior chamber. CB, ciliary body; I, iris; S, sclera.
|
|
| Treatment |
| In the Emergency Ward, latanoprost 0.005%, dorzolamide/timolol 22.3-6.8 mg/mL, and brimonidine 0.1% were administered in both eyes; the patient was given 500 mg oral acetazolamide to lower the IOP. Atropine 1% was also administered in both eyes. His IOP decreased to 37 mm Hg in each eye. Medications were maintained for 24 hours; IOP decreased to 15 mm Hg in each eye by the following day. His uncorrected visual acuity returned to his baseline of 20/20 in each eye by day 3, with improvement of his anterior chamber depth on biometry to 3.54 mm in the right eye and 3.56 mm in the left eye as measured by LENSTAR LS 900 Biometer. He was tapered off all drops and oral medications within 1 week. Serial UBM images showed resolution of the uveal effusions (Figure 2B). Automated static perimetry indicated no glaucomatous defects. |
|
| Differential Diagnosis |
| Bilateral angle closure secondary to uveal effusions can have multiple causes, including pharmacologic, inflammatory, neoplastic, vascular, and idiopathic mechanisms. Assessment for inflammatory and neoplastic etiologies is paramount in order to begin prompt and appropriate treatment. Structural etiologies, such as nanophthalmos, should also be investigated in order to prevent further unnecessary testing.(1) A thorough review of systems should be conducted, especially with regard to any recent new pharmacological intake. A commonly implicated pharmacologic agent resulting in uveal effusions is topiramate. Inflammatory conditions that can cause uveal effusions include posterior scleritis, Vogt-Koyanagi-Harada disease, and choroiditis. Despite the patient’s young age, it was deemed prudent to consider neoplastic causes, such as choroidal melanoma or ocular metastases. Hypertension, especially pulmonary hypertension, can also cause bilateral uveal effusions. Nanophthalmos can lead to spontaneous choroidal effusions and retinal detachments, albeit rarely. Lastly, uveal effusion syndrome, an idiopathic process, is a consideration of exclusion. It is important to understand the rarity of simultaneous bilateral primary angle closure and that the presence of bilateral angle closure should prompt investigation into secondary causes. |
|
| Diagnosis and Discussion |
Several pharmacologic agents are known to induce bilateral angle closure via uveal effusions, including topiramate, flucloxacillin, carbamazepine, venlafaxine, trimethoprim/sulfamethoxazole, hydrochlorothiazide, chlorthalidone, buproprion, indapamide, and escitalopram;(2-13) nevertheless, the pathophysiology of drug-induced bilateral angle closure via uveal effusions is unclear. Many of the medications implicated in bilateral uveal effusions have sulfa moieties, although this has not yet been postulated as a definitive cause in the pathophysiology of uveal effusions. One hypothesis attributes binding of the pharmacologic agents to choroidal tissue, resulting in an immune reaction and edema.(13) Another hypothesis focuses on serotonin, which is present in aqueous humor; serotonin receptors are expressed in the ciliary body and choroid.(14,15) Altered serotonin metabolism after drug exposure could contribute to the mechanism of uveal effusions in these cases.(13) To our knowledge, this is the first case report suggesting that a muscle relaxant may induce bilateral uveal effusions. Although we are limited in our report by the unidentified muscle relaxant, a common muscle relaxant, cyclobenzaprine, has known serotonergic effects, thus further supporting and aligning with the hypothesis of pharmacological precipitation of bilateral uveal effusions put forth by Murphy et al.(13)
Also as part of the assessment, a thorough dilated fundus examination is essential, with particular attention to assessing for vitritis and retinal/choroidal masses. Our patient had no masses or vitritis on examination, supporting our suspicion of a pharmacological or idiopathic etiology. Biometry measurements did not reveal a nanophthalmic eye. The patient did endorse taking a single dose of a new muscle relaxant provided by a friend 10 days earlier, although the specific drug was unknown, and the patient was unable to find this information. The timeline in this case is consistent with a drug-induced uveal effusion. A postviral etiology for the bilateral uveal effusions was initially considered. Mansour et al reported unilateral uveal effusion following H1N1 influenza infection;(16) however, the patient cited an episode of diarrhea only 1 day prior to his symptoms, making this etiology less likely.
Our report is unfortunately limited by the unknown type and dose of medication consumed by our patient, which was reportedly a muscle relaxant. Furthermore, he did not undergo a manifest refraction to objectively document the myopic shift as a result of the uveal effusions; nor did he receive a urine toxicology screen to definitely rule out other agents resulting in his presentation.
Once the diagnosis of pharmacologic-induced uveal effusion syndrome is established, treatment usually consists of medication cessation, topical cycloplegic agents to lower IOP via posterior rotation of ciliary process, and topical/oral antihypertensive drops. In these cases, the mechanism of secondary angle closure is not via pupillary block; therefore, a peripheral iridotomy is ineffective relieving the angle closure. However, argon laser peripheral iridoplasty has been successfully used as initial therapy.(8)
Literature Search
PubMed was searched without restriction on November 28, 2017, using the following terms: bilateral uveal effusion and muscle relaxant uveal effusion.
|
|
| References |
1. Abell RG, Kerr NM, Vote BJ. Bilateral nanophthalmic uveal effusion syndrome: clinical presentation and surgical management. Retin Cases Brief Rep 2013;7:386-90.
2. Chan KCY, Sachdev N, Wells AP. Bilateral acute angle closure secondary to uveal effusions associated with flucloxacillin and carbamazepine. Br J Ophthalmol 2008;92:428-30.
3. Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology 2004;111:109-11.
4. de Guzman MHP, Thiagalingam S, Ong PY, Goldberg I. Bilateral acute angle closure caused by supraciliary effusions associated with venlafaxine intake. Med J Aust 2005;182:121-3.
5. Postel EA, Assalian A, Epstein DL. Drug-induced transient myopia and angle-closure glaucoma associated with supraciliary choroidal effusion. Am J Ophthalmol 1996;122:110-2.
6. Reis GM, Lau OC, Samarawickrama C, Heydon P, Goldberg I. Utility of ultrasound biomicroscopy in the diagnosis of topiramate-associated ciliochoroidal effusions causing bilateral acute angle closure. Clin Experiment Ophthalmol 2014;42:500-1.
7. Roh Y-R, Woo SJ, Park KH. Acute-onset bilateral myopia and ciliochoroidal effusion induced by hydrochlorothiazide. Korean J Ophthalmol 2011;25:214-7.
8. Sbeity Z, Gvozdyuk N, Amde W, et al. Argon laser peripheral iridoplasty for topiramate-induced bilateral acute angle closure. J Glaucoma 2009;18:269-71.
9. Singer JR, Pearce ZD, Westhouse SJ, Siebert KJ. Uveal effusion as a mechanism of bilateral angle-closure glaucoma induced by chlorthalidone. J Glaucoma 2015;24:84-6.
10. Takusagawa HL, Hunter RS, Jue A, Pasquale LR, Chen TC. Bilateral uveal effusion and angle-closure glaucoma associated with bupropion use. Arch Ophthalmol 2012;130:120-2.
11. Végh M, Hári-Kovács A, Réz K, Tapasztó B, Szabó A, Facskó A. Indapamide-induced transient myopia with supraciliary effusion: case report. BMC Ophthalmol 2013;13:58.
12. Zelefsky JR, Fine HF, Rubinstein VJ, Hsu IS, Finger PT. Escitalopram-induced uveal effusions and bilateral angle closure glaucoma. Am J Ophthalmol 2006;141:1144-7.
13. Murphy RM, Bakir B, O’Brien C, Wiggs JL, Pasquale LR. Drug-induced bilateral secondary angle-closure glaucoma: a literature synthesis. J Glaucoma 2016;25:e99-105.
14. Sharif NA, Senchyna M. Serotonin receptor subtype mRNA expression in human ocular tissues, determined by RT-PCR. Mol Vis 2006;12:1040-7.
15. Martin XD, Brennan MC, Lichter PR. Serotonin in human aqueous humor. Ophthalmology 1988;95:1221-6.
16. Mansour DEAA, El-Shazly AA-F, Elawamry AI, Ismail AT. Comparison of ocular findings in patients with H1N1 influenza infection versus patients receiving influenza vaccine during a pandemic. Ophthalmic Res 2012;48:134-8.
|
|

