34 year old man with headache, vertigo, clumsiness, and unsteady vision
Digital Journal of Ophthalmology 1996
Volume 2, Number 1
August 14, 1996
Volume 2, Number 1
August 14, 1996
PMHx: Non contributory
Meds: None
SHx: Non contributory
FHx: Non contributory
Pupils: Normal OU, No APD
Color: Normal OU
External: Normal OU
Motility: Extraocular motility was abnormal . The patient had conjugate eye movements, and full ductions. There was a jerk nystagmus with the slow phase upgoing and the fast phase down-going. Fixation did not affect the nystagmus. The nystagmus was more prominent in down and lateral gaze, and dampened with up-gaze. Downward pursuit was abnormal.
Slit lamp examination: Normal OU
Fundus examination: Normal disc, macula, vessels OU
Visual fields: Goldmann visual fields and Amsler grid testing were normal
Neurologic Exam: The neurologic examination was notable for restricted range of motion of the neck, a diffuse increase in muscle tone, decreased proprioception of hands and feet bilaterally, dysmetria of the left arm, positive Romberg sign, ataxic gait, abnormal tandem gait, hyperreflexia with bilateral upgoing toes and ankle clonus.
Quicktime Video of Nystagmus (2.5 MB)
Download Quicktime Player
Download Quicktime Player
The prolonged and gradually progressive nature of the presented patient's symptoms, in the absence of risk factors for other entities in the differential, led to the suspicion of an anatomical process.
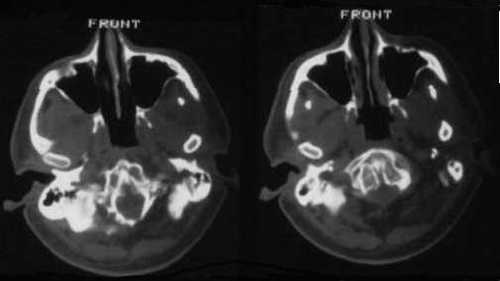
Figure 1
On CT scan, a bony abnormality of the clivus was evident.
On CT scan, a bony abnormality of the clivus was evident.
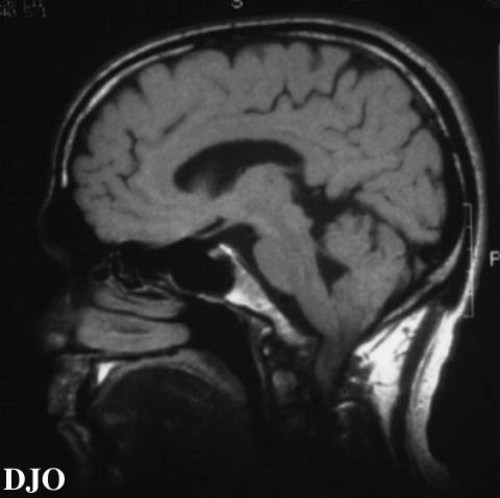
Figure 2
Figures 2-3. On MRI scan, the bony excrescence of the clivus was quite dramatic, with impingement of the medulla which rests its ventral surface on the clivus. The medulla was displaced 5 cm posteriorly, and the cerebellar tonsils were displaced posteriorly INTO the upper cervical spinal canal.
Figures 2-3. On MRI scan, the bony excrescence of the clivus was quite dramatic, with impingement of the medulla which rests its ventral surface on the clivus. The medulla was displaced 5 cm posteriorly, and the cerebellar tonsils were displaced posteriorly INTO the upper cervical spinal canal.
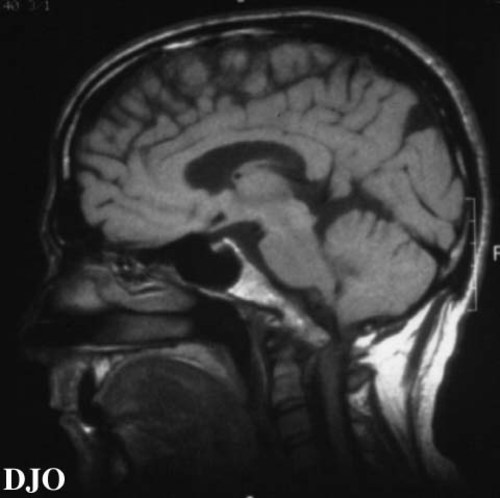
Figure 3
There are multipe etiologies of DBN, many of which are related to pathology at the cervicomedullary junction. These include anatomical anomalies of the posterior fossa, infarction, particularly of the vertebrobasilar system, cerebellar degeneration syndromes such as Friedreich's ataxia and olivopontocerebellar atrophy, and multiple sclerosis. 1 Structural lesions of the cervicomedullary junction include Arnold Chiari malformations2, syringomyelia, local and metastatic neoplastic processes, and bony abnormalities of the foramen magnum such as platybasia and basilar invagination.
Neoplastic disease can also produce a paraneoplastic cerebellar degenerative process with DBN3. The paraneoplastic cerebellar degeneration syndrome is commonly found with Hodgkin's lymphoma, as well as with breast, ovarian, and small cell lung cancer, and is characterized by a subacute onset of symmetical truncal and appendicular ataxia, dysarthria, and downbeat nystagmus.
Infections such as with the Herpes virus, the human immunodeficiency virus, and the Creutzfeldt-Jakob prion should also be considered. Drug toxicitity has also been associated with DBN, in particular the antiseizure medications carbamazepine and phenytoin, as well as lithium4, 5. Sporadic cases have also been reported among users of amiodarone and felbamate. DBN has also been reported with vitamin B12 and thiamine deficiency, alcohol use6, and magnesium deficiency. Congential DBN without associated abnormalities and DBN associated with blue cone monachromatism are rare.
Clinical Course
Given the patient's progressive and disabling symptoms of bidirectional vertical oscillopsia, vertigo, headache, dysmetria, and gait ataxia, as well as the precarious situation of HAVING the medulla rest on a protuberant bony abnormality, surgical intervention was recommended. After transoral decompression of the foramen magnum with removal of the clivus protuberance, there was considerable recovery. At a one year follow up examination, the patient reported oscillopsia only after vigorous exercise. Examination revealed trace nystagmus on lateral gaze, a normal gait, and normal reflexes.
Discussion
Downbeat nystagmus is a primary position vertical jerk nystagmus that is often accompanied by vestibular and cerebellar signs such as vertigo, ataxia, and oscillopsia. It is characterized by conjugate eye movements which occur in primary postion and are not affected by fixation. The nystagmus increases in amplitude in lateral gaze, is greatest in down and lateral gaze, and dampens in up gaze. There may be a torsional component in lateral gaze.
Studies have suggested that DBN is a pathologic central vestibular nystagmus caused by imbalance of the tonic activity of the vertical vestibulo-ocular reflex pathways. This disturbance of neural input and integration is thought to upset the usual balance of information that maintains fixation. It is hypothesized that an abnormal tone of the semicircular canal reflexes in the sagital plane causes a nystagmus of primary position with a slow vertical drift of the eye away FROM a target, and a fast saccade returning the eye to the target (7). For example, a reduction in a tonic inhibitory stimulus to the superior rectus or an increase in a tonic excitatory stimulus to the inferior rectus could cause an upward drift of the eye, and than a downward corrective saccade, to produce DBN.
The lesions causing the central vestibular imbalance of DBN are proposed to be either of the brainstem or of the vestibulo-cerebellum, which is comprised of the cerebellar flocculus and nodulus. These lesions lead to the disturbance of the connections between the cerebellum, medulla, and the anterior and posterior semicircular canals. Studies have shown that structural lesions of the fourth ventricle between the vestibular nuclei cause disruption of the tonic excitatory fibers to the inferior rectus muscles, and that a bilateral lesions of the flocculus lead to an increase in the tonic excitatory input to the superior rectus muscle (8). The diagnosis of DBN warrants an MRI to identify possible pathology of the cervicomedullary junction, and an investigation of possible iatrogenic, toxic, metabolic and paraneoplastic etiologies as suggestied by history and examination.
Treatment is directed at correction the underlying abnormality, whether it be via withdrawal of toxic drugs and substances, surgical decompression, treatment of neoplastic process, or correction of vitamin deficiency. Clonazepam has been used to decrease DBN and oscillopsia with some success, although it has side effect of drowsiness and fatigue (9). A recent study reports the efficacy of intravenous scopolamine in reducing nystgmus and oscillopsia and improving visual acuity and motion perception (10). This study's findings are supported by an earlier study by Dietertich which reports a transient worsening of DBN with injection of physostigmine, a cholinergic agonist (11). Dieterich also reports the efficacy of baclofen. Fresnel prisms and strabismus surgery to dampen the nystagmus by promoting a null point position have also been described (12).
Downbeat nystagmus with secondary oscillopsia is a potentially curable entity. Careful evaluation and therapeutic intervention may lead to improvement of disability and recognition of a potentially fatal disease.
2) Yee RD, Baloh RW, Honrubia V. Episodic vertical oscillopsia and downbeat nystagmus in a Chiari malformation. 102: 723-725. 1984
3) Anderson NE, Rosenblum MK, Posner JB. Paraneoplastic cerebellar degeneration: clinical-immunological correlations. Annals of Neurology 24:559-567. 1988
4) Halmagyi GM, Lessell I, Curthoys IS, Lessell S, Hoyt WF. Lithium-induced downbeat nystagmus. American Journal of Ophthalmology 107: 664-670. 1989
5) Corbett JJ, Jacobson DM, Thompson HS, Hart MD, Albert DW. Downbeating nystagmus and other ocular motor defects caused by lithium toxicity. Neurololgy. 39: 481-487.1989
6) Rosenberg ML. Reversible downbeat nystagmus secondary to excessive alcohol intake. Journal of Clinical Imaging. 7: 23-25. 1987
7) Gretsy M, Barratt H, Rudge P, Page N. Analysis of downbeat nystagmus. Archives of Neurology 43: 52-55. 1986
8) Zee DS, Yamazaki A, Butler PH, Gecer G. Effects of ablation of flocculus and paraflocculus on eye movements in primate. Journal of Neurophysiology 59: 370-393.1988
9) Currie JN, Matsuo V. The use of clonazepam in the treatment of nystagmus-induced oscillopsia. Ophthalmology 93: 924-032.1986
10) Barton JJS, Huaman AG, Sharpe JA. Muscarinic antagonists in the treatment of acquired pendular and downbeat nystagmus: a double-blind randomized trial of three intravenous drugs. Ann Neurol 35: 319-325. 1994
11) Jakobiec FA, Folberg R, Iwamoto T. Clinicopathologic characteristics of premalignant and malignant melanocytic lesions of the conjunctiva. Ophthalmology 96:147-166. (1989)
12) Jakobiec FA, Rini FJ, Fraunfelder FT, Brownstein S. Cryotherapy for conjunctival primary acquired melanosis and malignant melanoma. Ophthalmology 95:1958-1070. (1989)
13) Folberg R, McLean IW, Zimmerman LE. Conjunctival melanosis and melanoma. Ophthalmology 91:673-678. (1984)